Experiment 4
Chemical Changes: Reactions of Copper
Optional protocol using a penny as the source of copper (at the
discretion of your instructor).
Instead of using a piece of copper wire, as described in the printed protocol,
you will use a penny (you must use a 1970 or later penny). Current pennies are
made from a piece of zinc coated with a thin layer of copper. The modern penny
is not 100% pure copper as it was prior to the 1960's. You will dissolve the
entire penny in the concentrated nitric acid.
To do this experiment follow the modified protocol shown below:
1. Cu (s) + 4 HNO3 (aq)
→ Cu(NO3)2 (aq) + 2 NO2 (g) +
2 H2O (l)
- Place a single penny (you should have determined its mass) into a 250-mL
Erlenmeyer flask.
- Add 20 mL concentrated nitric (HNO3) acid.
- Let the penney completely dissolve, or if you believe all the copper has
dissolved, you can remove the zinc core using a pair of forceps.
- Add about 20 mL of DI water before proceeding with the next step.
2. Cu(NO3)2 + 2 NaOH (aq)
→ Cu(OH)2 (s) + 2 NaNO3
(aq)
- Add about 50 mL of 6 M NaOH in small increments, with stirring.
- The bluish Cu(OH)2 solid also contains the Zn(OH)2,
which is white.
- Using litmus paper, check the pH to make certain the liquid is basic (red
litmus turns blue).
3. Cu(OH)2 (s)
→ CuO (s) + H2O (l)
- Heat your sample, with stirring, until all the blue color disappears, leaving
a grey slurry (your mixture will contain both the black CuO and the white
ZnO).
- Collect your sample using vacuum filtration and a Büchner funnel (you
must use #2 filter paper, not #3 filter paper).
- Remove the filter from your funnel and place in the bottom of a 250-mL beaker
(with the solids on the top side).
4. CuO (s) + H2SO4 (aq)
→ CuSO4 (aq) + H2O
(l)
- Add about 20 mL of the 3 M H2SO4 and let it dissolve,
with occassional swirling of the beaker to completely dissolve the solid material.
- When all the solid has been dissolved, the solution should be a light blue
in color.
- Add about 40 mL of DI water, swirl, and then remove the filter paper.
Cover your sample with a layer of parafilm and place on the cart for storage
until the next lab period. The blue color of the liquid is due to the Cu2+
ion. The Zn2+ ion is colorless, but is also present. (If your
liquid has pieces of the filter paper in it, you must do a filtration prior
to doing the Mg reduction step.)
During the second day of your lab, you will then reduce your Cu2+
to produce Cu metal using Mg. Don't worry about the Zn2+ ion which
also gets reduced (producing Zn metal), because any Zn metal produced will either
react with the sulfuric acid or it will help to reduce the Cu2+ into
the Cu metal. By doing this reduction step (starting with Mg and your solution
of ions) you will produce only Cu metal, and all the zinc and magnesium will
be converted, or stay, as ions.
5. CuSO4 (aq) + Mg (s)
→ Cu (s) + MgSO4 (aq)
- If your liquid has pieces of the filter paper in it, you must do a Büchner
funnel vacuum filtration prior to adding the Mg filings.
- Pour the clear filtrate into either a 400-mL or 600-mL beaker.
- Add the required amount of Mg filings (see published protocol for amount)
to your beaker, a few pieces at a time.
- Continue to add more Mg until all the blue color in the liquid is gone (Cu2+
is blue in solution; therefore, when it is all reduced to Cu, the blue color
will disappear).
- If any bubbles are apparent, then all the Mg has not yet been reacted. If
in doubt, add up to 10 mL of 3 M H2SO4 to assist in
the dissolving. Do not filter until no more gas (bubbles) is produced.
To collect your copper do the following:
- Filter the final mixture (the liquid must be colorless, but will continue
the reddish or black-looking copper) to collect your Cu metal via Büchner
funnel and vacuum filtration.
- Wash the solid material with copious amounts of water to remove any acid
from the filter paper (residual acid will cause the filter paper to turn black
when heated).
- Keep the vacuum on for at least 5 min after your final wash to remove as
much water as possible.
- Dry your Cu metal (along with a preweighed piece of filter paper) in the
drying oven (use a pre-weighed evaporating dish or watch glass to put your
filter in).
Weigh your dry Cu sample and determine the amount of copper present (remember
to subtract the mass of the pre-weighed evaporating dish and filter paper).
Determine the percent yield of Cu based on the mass of the original penny using
the %Yield formula given below.
To obtain a value for %Yield, you need to know the
Theoretical Value you should obtain, based on balanced equations,
and if 100% of the reactant(s) is converted to product. After obtaining
your Experimental Value, divide it by the Theoretical Value,
then multiply by 100 to get %Yield.
|
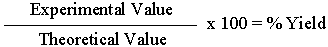 |
Questions:
- Why does the copper not dissolve in the H2SO4 mixture
in the last step?
- Practice doing the oxidation-reduction reaction for Cu combining with concentrated
HNO3, which is the first reaction. (You will see this reaction
again on quizzes, the lab exam and the regular exam.)
- If you used dilute HNO3, instead of the concentrated acid used
in this experiment, you would produce NO (g) instead of the NO2
(g). Show the correct oxidation-reduction equation for the production of NO.
Reagents needed for use of a penny (per group)
- 20 mL concentrated nitric acid
- 50 mL 6 M NaOH
- 40 mL H2SO4 (20 mL to dissolve solid CuO and 20 mL
to dissolve excess Mg after last step)
- Use only #2 Büchner filter paper, do not use #3 paper as it is too
thick and gets clogged during the CuO filtration
Go To Experiment: 1
2 3
4 5
6 7
8 9
10 11
12
Return to Chem110 Experiments Index
![]()