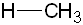
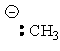
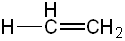
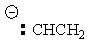
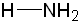
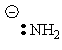
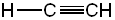
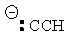
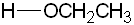
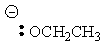
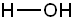
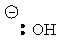

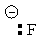
| Conjugate acid | Formula | Structure | pKa | Conjugate base | Name |
|---|---|---|---|---|---|
| Methane | CH4 |
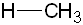 |
50-60 | 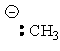 |
Methyl |
| Ethene | CH2CH2 |
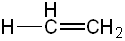 |
45 | 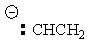 |
Ethenyl |
| Ammonia | NH3 |
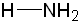 |
36 |  |
Amide |
| Acetylene (ethyne) | C2H2 |
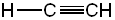 |
26 | 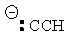 |
Acetylide |
| Ethanol | C2H5OH |
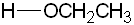 |
16 | 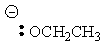 |
Ethoxide |
| Water | HOH |
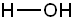 |
15.7 | 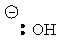 |
Hydroxide |
| Ammonium | NH4+ | H—NH3 | 9.8 | : NH3 | Ammonia |
| Hydroflouric acid | HF |
 |
4 | 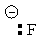 |
Flouride |
Use this table to predict which conjugate base will favorably react with which conjugate acids. Pay attention to the pKa values shown. As a general rule, the conjugate base of any acid will react with, and remove, the proton (H+ ion) from any conjugate acid that is stronger than the conjugate acid from which the conjugate base you are looking at was derived from.
In the chart above, the conjugate base will remove a proton from every conjugate acid that is below it in the table.
Examples:
When I write — quantitatively — I mean that there is enough difference in the pKa values (of the conjugate acids) to remove greater than 99% of the protons from the acids below them. A difference of 2 pKa values will allow the conjugate base coming from the weaker acid will remove >99% of protons from the stronger acid.