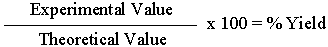%Yield
To obtain a value for %Yield, you need to know the Theoretical Value you should obtain, based on balanced equations, and if 100% of the reactant(s) is converted to product. After obtaining your Experimental Value, divide it by the Theoretical Value, then multiply by 100 to get %Yield.