%Yield
To obtain a value for %Yield, you need to know the Theoretical Value you should obtain, based on balanced equations, and if 100% of the reactant(s) is converted to product. After obtaining your Experimental Value, divide it by the Theoretical Value, then multiply by 100 to get %Yield.
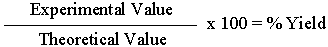
%Error
To obtain %Error for a measurement, you need to know the Theoretical and Experimental values for your measurement, or set of calculations. Use this formula to obtain the %Error, which is always a positve (absolute) value.

Theoretical Yield or Value
In order to obtain a value for the Theoretical Yield for a reaction you must know how many moles or grams of a chemical you start with. For organic chemistry, you need only look at the organic part of the product to see what the corresponding starting compound would be. For example, if you are carrying out a reaction to produce bromobenzene, you must have the following reactants: benzene, FeBr3 catalyst, and Br2. Unless the reaction is woefully deficient in either FeBr3 or Br2, only be concerned with the amount of benzene. What you would need for this reaction is only the mass of benzene. You really would not even need to know the moles of benzene or the moles of bromobenzene produced. Using the balanced equation, for each mole of benzene (78 g/mol) you will produce one mole of bromobenzene (157 g/mol). Therefore, 78 g of benzene will produce 157 g of bromobenzene. If you know how much benzene you start with (e.g., 5.7 g benzene in example here), then the theoretical yield of bromobenzene ("X" in the example shown) is simply a ratio to solve algebraically. It's that easy.

![]()