ISOSTATIC EQUILIBRIUM
Pre-Lab Exercise
PART 1: VOLUME, MASS, & DENSITY
Read the information below to answer the pre-lab questions.
Density is a
measure of mass per unit volume.
To use water as an example, a gallon (a unit of volume) weighs
about 8.33 pounds (a unit of mass). Therefore, the density of water is 8.33 pounds per gallon. We can use any measurement of mass and/or
volume to express density. Water’s density is also 62.4 pounds per cubic
foot (62.4 lbs/ft3), 1.0 kilogram per liter (1.0 kg/L), or
1.0 gram per cubic centimeter (1.0 g/cm3), which is the same
thing as 1.0 gram per milliliter (1.0 g/mL).
In this lab exercise, we will use the standard Metric System unit for
density, which is grams per cubic
centimeter: g/cm3
To measure the density of
something in g/cm3, we
need to measure both its mass in grams
and its volume in cubic centimeters. Density is always equal
to mass divided by volume, not the other way around. A common error students make is to calculate
volume divided by mass, which is the inverse of density.
Measuring mass is easy; you
can just weigh an object on a scale.
When you stand on a bathroom scale, you probably measure your mass
(weight) in pounds. But you could
just as accurately express it in grams, although it will be a large
number: mass in pounds x 454 = mass in grams.
Measuring volume is more
involved. In the lab, you will measure
volume in two ways: by linear dimensions and by water displacement.
Volume
from linear dimensions:
Measuring volume with linear dimensions works
for any object that is geometrical: cubes, spheres, cylinders, cones, and so
forth. For example, if you have a
rectilinear object—like a brick or a rectangular block of wood—you can measure
its volume by measuring its length, width, and thickness and multiplying the
three together. For example, this object
has a volume of 25cm x 9cm x 8cm = 1800
cm3

Volume
from water displacement:
If you want to know the volume of an object that has an irregular shape—like
your body, or a piece of rock—then the easiest method is water
displacement. An object immersed in
water will displace a volume equal to the object’s volume. For example, if you filled a bathtub
precisely to the brim and were then able to catch all the water that overflowed
when you immersed yourself, the volume of the displaced water would be equal to
your body volume.
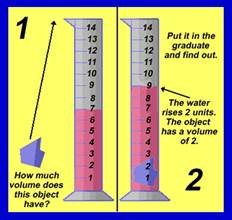
In the lab, we will measure the
volume of rock samples by measuring the amount of water they displace in a
graduated cylinder, like this:
------------------------------------------------------------------
PART 2: ISOSTATIC EQUILIBRIUM DEMONSTRATION
Watch and listen to the short video below to answer the pre-lab
questions for this section.
Note: You can turn CAPTIONS on
or off by clicking “CC” at the bottom right of the video screens. Also, you can
view the videos at higher resolution by choosing a better quality option from
the menu at the bottom right of the screen.
Video
by Keith Meldahl