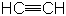.
.
Functional Group Containing Compounds
| Compound |
Examples |
Acceptable Name |
Characteristic Reactions |
| Alkyl halides |
CH3CH2Cl |
Chloroethane or ethyl chloride |
Nucleophilic subsitution; elimination |
| Alkenyl halides |
CH2=CHCl |
Chloroethene or vinyl chloride |
Electrophilic addition; elimination |
| Aryl halides |
C6H5Cl |
Chlorobenzene |
Electrophilic & nucleophilic aromatic substituion |
| Alcohols |
CH3CH2OH |
Ethanol or ethyl alcohol |
Dehydration; esterification |
| Phenols |
C6H5OH |
Phenol |
Electrophilic subsitution |
| Epoxides |
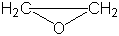 |
Ethylene oxide or oxirane |
Nucleophilic ring opening |
| Aldehydes |
CH3CHO |
Ethanal or acetaldehyde |
Nucleophilic addition to carbonyl |
| Ketone |
CH3COCH3 |
2-Propanone or acetone |
Nucleophilic addition to carbonyl |
| Carboxylic acids |
CH3COOH |
Ethanoic (acetic) acid |
Acid ionization; ester formation |
| Acyl halides |
CH3COCl |
Ethanoyl (acetyl) chloride |
Nucleophilic acyl subsitution |
| Acid anhydrides |
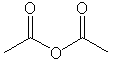 |
Ethanoic (acetic) anhydride |
Nucleophilic acyl subsitution |
| Esters |
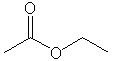 |
Ethyl ethanoate (acetate) |
Nucleophilic acyl subsitution |
| Amides |
CH3CONHCH3 |
N-Methylethanamide or N-methylacetamide |
Nucleophilic acyl subsitution |
| Amines |
CH3CH2NH2 |
Ethanamine or ethylamine |
Nitrogen (base) as nucleophile |
| Nitriles |
CH3CN |
Ethanenitrile or acetonitrile |
Nucleophilic addition |
| Nitro comps. |
C6H5NO2 |
Nitrobenzene |
Reduction of nitro to amine |
| Thiols |
CH3CH2SH |
Ethanethiol |
Oxidation to acids or disulfides |
| Sulfides |
CH3CH2SCH2CH3 |
Diethyl sulfide |
Alkylation or oxidation |
.
Organic Compounds, and the Types of Reactions They Undergo
| Compound |
Examples |
Acceptable Name |
Characteristic Reactions |
| Alkanes |
CH3CH3 |
Ethane |
Free-radical substitution |
| Alkenes |
CH2=CH2 |
Ethene or ethylene |
Electrophilic addition |
| Alkynes |
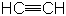 |
Ethyne or acetylene |
Electrophilic addition |
| Dienes |
CH2=CHCH=CH2 |
1,3-Butadiene |
Electrophilic addition |
| Arenes |
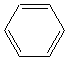 |
Benzene (C6H6 ) |
Electrophilic subsitution |
| Alkyl halides |
CH3CH2Cl |
Chloroethane or ethyl chloride |
Nucleophilic subsitution; elimination |
| Alkenyl halides |
CH2=CHCl |
Chloroethene or vinyl chloride |
Electrophilic addition; elimination |
| Aryl halides |
C6H5Cl |
Chlorobenzene |
Electrophilic & nucleophilic aromatic substituion |
| Alcohols |
CH3CH2OH |
Ethanol or ethyl alcohol |
Dehydration; esterification |
| Phenols |
C6H5OH |
Phenol |
Electrophilic subsitution |
| Epoxides |
 |
Ethylene oxide or oxirane |
Nucleophilic ring opening |
| Aldehydes |
CH3CHO |
Ethanal or acetaldehyde |
Nucleophilic addition to carbonyl |
| Ketone |
CH3COCH3 |
2-Propanone or acetone |
Nucleophilic addition to carbonyl |
| Carboxylic acids |
CH3COOH |
Ethanoic (acetic) acid |
Acid ionization; ester formation |
| Acyl halides |
CH3COCl |
Ethanoyl (acetyl) chloride |
Nucleophilic acyl subsitution |
| Acid anhydrides |
 |
Ethanoic (acetic) anhydride |
Nucleophilic acyl subsitution |
| Esters |
 |
Ethyl ethanoate (acetate) |
Nucleophilic acyl subsitution |
| Amides |
CH3CONHCH3 |
N-Methylethanamide or N-methylacetamide |
Nucleophilic acyl subsitution |
| Amines |
CH3CH2NH2 |
Ethanamine or ethylamine |
Nitrogen (base) as nucleophile |
| Nitriles |
CH3CN |
Ethanenitrile or acetonitrile |
Nucleophilic addition |
| Nitro comps. |
C6H5NO2 |
Nitrobenzene |
Reduction of nitro to amine |
| Thiols |
CH3CH2SH |
Ethanethiol |
Oxidation to acids or disulfides |
| Sulfides |
CH3CH2SCH2CH3 |
Diethyl sulfide |
Alkylation or oxidation |
.
Some Commonly Encountered Groups
| Group |
Name |
Group |
Name |
| CH3 — |
Methyl |
CH3CH2 — |
Ethyl |
| CH3CH2CH2 — |
Propyl or n-propyl |
(CH3)2CH — |
1-Methylethyl or isopropyl |
| CH3CH2CH2CH2 — |
Butyl or n-butyl |
|
CH3CHCH2CH3
|
1-Methylpropyl or sec-butyl |
| (CH3)3C — |
1,1-Dimethylethyl or tert-butyl |
(CH3)2CHCH2 — |
2-Methylpropyl or isobutyl |
| (CH3)3CCH2 — |
2,2-Dimethylpropyl or neopentyl |
CH2=CH — |
Ethenyl or vinyl |
| CH2=CHCH2 — |
2-Propenyl or allyl |
|
CH2=CCH3
|
1-Methylvinyl or isopropenyl |
|
CH3C=O
|
Ethanoyl or acetyl |
 |
Phenyl (C6H5 — ) |
 |
Phenylmethyl or benzyl
(C6H5-CH2 — ) |
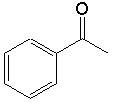 |
Benzenecarbonyl or benzoyl
(C6H5-CO — ) |
|
|
|
|