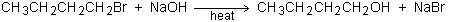
During the first three labs, Experiments Ia, Ib, and Ic, we will be performing several different separation techniques:
Each of these separation techniques will be used at other times during the lab this semester. These experimental protocols serve only as an introduction to these procedures.
We will also begin our use of computers for access information online and to examine organic structures. You are expected to use several sources for chemical information, including texts, reference manuals and chemical catalogs to get the information you will need in order to analyze and separate the chemicals used in this experiment. You will also use www.chemfinder.com, which is an online source of useful chemical information. Computers in the lab are already setup to use at chemfinder.com. If prompted for a Username, enter mccchem. The password is mccmcc. You can also use chemfinder.com at home, and either set up your own username and password, or you can use the ones listed here.
Part A: Using various reference sources (e.g., texts, reference manuals, catalogs, and the Internet) you will need to find information (formula, molecular weight, solubility, density, etc.) for the compounds used in Experiments Ia, Ib, and Ic (plus the chemicals used in other experiments). You will also have an introduction to the computer program ChemDraw, and will draw a simple hydrocarbon using this program.
Part B: You will separate of solids using different techniques. You and your lab partner will determine which techniques to use. Please check with the instructor to make certain your procedures are safe and practical.
Techniques required for these experiments are from The Organic Chem Lab Survival Manual, by Zubrick: Chap 1 (all); Ch 3 (pp 26-31, 34-41); Ch 4 (all); Ch 9 (all)
Organic chemistry can be divided into three general areas: Synthesis (making new compounds); Analysis (determining what compound(s) you have in a particular sample); and Natural Products chemistry (extracting organic compounds from biological sources, and determining the structure and properties of these natural products). All of these areas of laboratory activity use separation techniques on a daily basis.
In the real world, including the organic chemisty laboratory, it is almost always messier and more complicated than a lecture or textbook makes it seem. For instance, the synthesis of 1-butanol from 1-bromobutane, as given in the chemical reaction equation, looks pretty straight-forward, but if you are actually planning to carry out this synthesis in the chemistry laboratory, you will have to do a lot of planning!
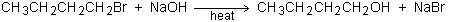
For instance, what solvents will you use? How will you heat the mixture safely and efficiently? How much of each reagent will you use? And, one of the most important questions: How will you separate the desired product from the mess of solvent, unreacted starting materials, by-products, and other impurities?
The second part of today's experiment (Part B) will introduce you to the general concept of separations, as used in the organic chemistry laboratory. You and your partner will be given a small sample of a mixture containing aspirin, NaCl and silica (silicon dioxide). You will need to figure out how to isolate the two solid components by separating them from the liquids. The types of separations you will carry out are simple (you have probably used these techniques in previous chemistry courses), but it is the important to learn how to a separation scheme to isolate one or more compounds. You will take advantage of physical and chemical properties of these compounds.
Part A will serve as an orientation to the Laboratory Notebook Format, including an introduction to finding the properties of chemicals in the various reference works generally available. Do not write anything in your notebook before coming to lab! You will work in groups, in the lab, to find the information you need, and then to write the Preparation assignment for this experiment.
Get to Know Your Equipment: Before you begin an experiment be sure you know what equipment you will be using (including what it looks like, how to use it, how to clean it, etc.). If the Experimental Protocol does not describe it well enough, look in The Organic Chem Lab Survival Manual for more information. If none of the material available to you outside of class describes the equipment and its use satisfactorily, your instructor will probably talk about it during the pre-laboratory lecture. If you still need more information, ask questions before you start the experiment. Take notes during the pre-lab lecture so that you will have the information handy when you need it.
Most of the equipment you will use during this course will be in your laboratory drawer, your organic chemistry glassware kit, or located in one of the drawers or cabinets in the laboratory, near the computers. If a listed item is not in your drawer or kit, your instructor will tell you where in the lab it is located. In some cases, you will have to check out equipment from the laboratory stockroom. In all cases, it is your responsibility to clean equipment and glassware before you return it to its proper location. Damaged or broken items must be paid for by you before you can check out of the lab.
Safety: This is a "no chemical" exercise. You do not need to wear goggles or other safety equipment, unless others in the lab are currently working with chemicals.
Have your copy of the "Format for Laboratory Experiment Assignments" handy while you work on part A. Use the available reference books (described in Zubrick, chapter 3), Material Safety Data Sheets, and online sources (http://www.chemfinder.com) to find the information you need to fill out the Table of Substances and the Toxicities and Hazards section for your Lab Experiment. The compounds you need to include are listed below. Find some information (e.g., melting point, boiling point, refractive index, solubity, molecular weight, density, toxicity, and any other value that is important) for at least six of the following compounds that will be used in the upcoming experiments (Ia, Ib, and Ic).
|
|
|
|
|
You must use at least two reference sources for each of the chemical that you look up. For example, for aspirin, you could report data from the Merck Index and from ChemFinder, and these could be your two sources for aspirin. You also need to print the first page for one of the above listed chemicals using ChemFinder from the Internet (print only page one from the Print menu). You should print the ChemFinder first page for only one of the chemicals. List several different physical constants and information for the compounds you select, but you are not writing an encyclopedia, so you don't need to write down everything available. This exercise is to acquaint you with using the reference sources, not to have you write a paper on these compounds.
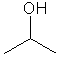
In Experiment III, you will use ChemDraw and Chem3D more extensively. However, to get your started, you will be introduced to ChemDraw. You will use this program to draw the chemical structures for three easy compounds: 2-propanol, ethanol and benzoic acid, as shown below.
 |
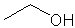 |
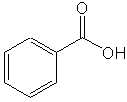 |
| 2 propanol | ethanol | benzoic acid |
Safety: Diethyl ether is a highly flammable, volatile, toxic liquid--wear gloves whenever you handle it, and NO FLAMES ALLOWED! Avoid breathing its vapors, it is an anesthetic!
Each group will obtain a vial containing a mixture of three solids: NaCl, aspirin (acetyl salicylic acid) and silica. It is up to the group to determine how you can isolate these two solids from each other using techniques based on the chemical or physical properties of these chemical, such as solubility, filtration, density, etc. We will some procedures in class, but it is up to each group to come up with their own final procedure, which allows for quantitative recovery of the different compounds.