|
Ibuprofen |
Acetaminophen |
Acetylsalicylic acid |
Phenacetin |
Caffeine |
|
|
|
|
|
|
The purpose of this experiment is to determine the components of some over-the-counter medicines using TLC. Some of the chemicals that you may be using in this experiment are shown below. Notice that all these pain relievers (called NSAIDS, for non-steroidal antiinflammatory drugs), contain a benzene ring; the caffeine enhances the pain-relieving effect.)
|
Ibuprofen |
Acetaminophen |
Acetylsalicylic acid |
Phenacetin |
Caffeine |
|
|
|
|
|
|
Chromatography is a general method of separation and analysis which uses differences in physical properties, often based on polarity, to separate compounds. Size or charge can also be used to separate molecules. We will use TLC to determine which compounds are present in several over-the-counter medications. The compounds which we will analyze may include: acetylsalicylic acid (aspirin), phenacetin, ibuprofen (MoltrinTM), acetamenophen (TylenolTM), and caffeine.
Safety: Acetylsalicylic acid, ibuprofen, and acetamenophen are toxic and irritants -- wear gloves while handling them. Phenacetin is a cancer suspect agent (potential carcinogen) -- wear gloves while handling it, and take special care not to ingest or inhale its powder. Caffeine is toxic in large quanties -- do not ingest it. Methanol, which is poisonness and can lead to blindness if ingested, and acetone are both flammable -- wear gloves and do not inhale vapors while using them. Dichloromethane (used in the second lab period) is volatile and toxic.
For this particular experiment, at the option of your instructor, you may do thin layer chromatography of ink samples. The basis for this option is that ink forensics is an important field in establishing whether documents are authentic or not. In addition, by determining the make up of an ink sample one can determine where the ink came from. So, before setting up your laboratory notebook, ask your instructor whether you will be doing the procedure detailed below, using TLC to analyze different pain relievers, or the ink TLC. If using the ink TLC lab, your instructor will describe what will be done during the first day of the lab, and you will design your own experiment to do ink TLC. The actual experiment will be done during the second day of the lab.
The first step is to make a number of spotters, as described in Zubrick (pp. 257-258). You will need at least six -- one for each of the known compounds and one for each of the unknowns (you can re-use each spotter as long as it is for the same chemical). Since this step uses Bunsen burners, and TLC uses flammable solvents, no one will be allowed to use any of the organic solvents until all burners are turned off. Therefore, make all the spotters you will need at the beginning of class.
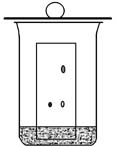
Make your own chromatography developing chamber using a 150-mL beaker (outlined in Zubrick, p. 260; an image is shown below).
|
It
is important to saturate the air in the chamber to obtain better
chromatography results. The filter paper helps to saturate
the chamber by allowing more surface area for the acetone to impregnate
and evaporate. Keep a lid on the chamber, such as a watch
glass or a larger beaker turned upside down while you prepare your TLC
plates. You will use acetone as the chromatography solvent.
Obtain about 20 mL of acetone, and keep it covered to prevent
evaporation. Pour enough acetone into your chromatography
chamber to produce a height of about 5 mm, but not higher.
|
 |
Obtain about 0.1 g samples of acetylsalicylic acid, phenacetin, and caffeine. Do not take more than 0.1 g of any chemical. Obtain one tablet for each of the three medications, that will be the unknowns. Use a mortar and pestal to grind the pill into powder. Use no more than 0.1 g of any chemical. You can use much less. To your 0.1-g samples, add about 5 mL of methanol. Some of your samples may not fully dissolve, but do not add more solvent (you can warm your sample using a hot water bath , using a hot plate, if desired). Regardless, there will be enough chemical dissolved to spot and detect using the UV lamp, which you can monitor prior to chromatography. Keep your spotters in the tubes. When storing your solutions until the next lab period, use Parafilm to seal the tubes and store on the chemical cart.
Each laboratory pair will use one 10 cm x 10 cm silica gel TLC plate (standard plates are 20 cm x 20 cm), which you can cut into 8 smaller pieces (each 2.5 cm x 5 cm), using scissors. You should mark these plates using only pencil (not ink, since the solvents dissolve ink), but be very careful not to touch the adsorbent (silica gel) with your fingers! Each group will use 4-8 of these smaller plates (if more are needed, cut them only when you are ready to use them, to avoid waste). The first TLC plate will be used to determine the Rf values for each of the three standard compounds, which you can do with one TLC plate. Prepare one plate with all spots of all three compounds, side by side. Use the procedure given in Zubrick to:
The next TLC plate will be used to determine how many substances there are in each pill, to measure the Rf values of each component, and to try to indentify the major chemical. Prepare one TLC plate with spots from each of the three pill samples, side by side. Develop and visualize the plate, and calculate the Rf values of each spot. Use this information to match the spots in the pill samples to the known compounds. In any case where there is confusion, run another plate with just the one pill and the one or two compounds that are possible components of the pill.
Today you will perform an additional set of experiments using TLC. If you have not finished the previous lab's experiment, or still need to repeat part of it, go ahead and finish Part A first. Then, you will be able to complete Part B today.
Instead of using a single chromatography solvent (e.g., acetone), as in Part A, you will use two additional solvents or solvent mixtures today. For example, you might try using dichloromethane alone and seeing if there is a difference in migration of the different standard chemicals (aspirin, phenacetin, and caffeine). For a second solvent, try using a 50:50 mixture of dichloromethane and acetone together as the chromatography solvent.
Therefore, using your chromatography standards determine which of the three solvents (acetone, dichloromethane or the 50:50dichloromethane-acetone) is the best solvent for TLC of the chemicals used in this experiment. You should be able to determine which solvent gives you the most useful information, which is usually based on the efficient separation of the different chemicals, based on optimal Rf values, size of spots, etc.
You should include at least 1-2 of your actual TLC plates, with appropriate markings on them, in your laboratory notebook that you hand in for credit. You can sketch TLC diagrams for your other experiments as well, so that your laboratory notebook is well documented with visual results.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Acetylsalicylic acid (aspirin) | 136.15 | 1.6338 g | 12.00 | -12 | 198-199 | 1.094 | 1.5170 | msds |
| Phenacetin | msds | |||||||
| Caffiene | msds | |||||||
| Dichloromethane | msds | |||||||
| Acetone | msds | |||||||
| Methyl alcohol | 181.14 | --- | --- | 78-80 | 279 | --- | --- | msds |
| Compound | (g/mol) | (grams or mL) | (10-3 M) | (oC) | (oC) | (g/mL) | ηD | msds |