The formation of new carbon-carbon bonds is one of the most important aspects of synthetic organic chemistry. Many reactions, such as the Grignard reaction and the use of acetylide ions in SN2 reactions, have been developed with this one goal in mind. One problem associated with most of these C—C bond-forming reactions is the necessity for exotic conditions — the Grignard reaction requires completely water-free conditions and acetylide ions must be generated in liquid ammonia solution.
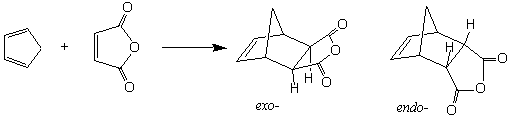
When a synthetic sequence calls for the formation of a ring of carbon atoms, this problem is compounded. Fortunately, the formation of six-membered carbon rings is much simpler than it would first appear. As described in Organic Chemistry by Carey (Section 10.12), the Diels-Alder reaction was discovered in 1928. This reaction forms a six-membered ring from two pieces: a conjugated "diene" (which provides four of the ring atoms) and a "dieneophile" (which provides two of the ring atoms). The main requirements for these species are that the conjugated diene must be somewhat electron rich (which is normally the case for dienes) and able to achieve the s-cis conformation, and that the "dieneophile" have a two-atom π system that is relatively electron poor.
In this experiment you will react cyclopentadiene (the diene) with maleic anhydride (the dienophile) to produce the bicyclic compound, endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride. The endo-adduct is formed exclusively. Why?

One important aspect of this experiment is that the cyclopentadiene must be freshly distilled within one day before the reaction is carried out. Why? Answer at end of experiment!
Safety: Cyclopentadiene (and its dimer, dicyclopentadiene) is an irritant, is flammable, has an unpleasant odor and is harmful if inhaled — avoid breathing its vapors. No flames will be allowed in the lab. Wear gloves while handling these chemicals. Dispense and use these chemicals in the hood to minimize inhalation hazards. Maleic anhydride is corrosive and toxic--wear gloves while handling it. Be sure to wash your gloves and your hands after handling it. Ethyl acetate is a flammable liquid and an irritant. Ligroin is a flammable liquid and toxic. No flames allowed, and wear gloves while handling solvents if possible, and avoid inhaling their vapors.
You will prepare your own cyclopentadiene using a fractional distillation setup (in the hood). The reflux column will be another condenser column (or you can use a fractional distillation column) using air to cool, if compressed air is available. Two groups, working together, will distill the amount of dicyclopentadiene needed for your experiments. Collect the cyclopentadiene into a receiver flask cooled to 0oC using an ice-water bath. Assemble a fractional distillation setup as used previously in lab, or as described above. This apparatus will use a 100-mL round-bottom distilling flask and a 50-mL round-bottom receiving flask. Place the receiving flask in a beaker filled with crushed ice and a little water so that the volatile product, cyclopentadiene, will be cooled to about 0oC, which prevents formation of the cyclopentadiene dimer.
Place about 30 mL of technical-grade dicyclopentadiene (about 85% pure) in your distillation flask, add several boiling stones, and attach the flask to your fractional distillation setup. Turn on the condenser water and heat the dicyclopentadiene until it refluxes briskly. (Why is air used to cool the reflux column?) The monomeric cyclopentadiene should start to distill within a few minutes. Continue to heat the dimer reagent to promote fairly rapid distillation, but do not allow the distillation temperature to exceed about 45oC, which is slightly above the boiling point of cyclopentadiene (40-43oC). It will take at least 30-40 minutes to collect the 12-mL sample of cyclopentadiene which is needed for your two reactions (two groups are distilling the cyclopentadiene together, but will perform the experiment separately).
When the cyclopentadiene has been collected, stop the distillation and discard the unused residue from the reaction flask, into the liquid waste container (boiling stones go into solid waste). If the cyclopentadiene is cloudy as a result of moisture condensing in the receiver, add a few pieces of anhydrous sodium sulfate (or anhydrous magnesium sulfate).
Instead of preparing your own cyclopentadiene (which often takes about 90 minutes), the stockroom may distill the dicyclopentadiene to produce the cyclopentadiene monomer for use during the lab. If this is done, the distillation should start about 30 minutes prior to its use in the lab to ensure that no dimer is reformed. The boiling point of the dicyclopentadiene is 170oC, whereas the cyclopentadiene monomer is about 41oC. Therefore, monitoring for temperature, collection of monomer can be achieved (into an ice-cold beaker) immediately prior to being used. This latter procedure is extremely efficient in providing cyclopentadiene monomer for reaction.
When the cyclopentadiene has been collected, stop the distillation and discard the unused residue from the reaction flask, into the liquid waste container (boiling stones go into solid waste). If the cyclopentadiene is cloudy as a result of moisture condensing in the receiver, add a few pieces of anhydrous sodium sulfate (or anhydrous magnesium sulfate).
Decant the dry cyclopentadiene from the collection flask or beaker use in the experimental procotol described below:
Using your dry crystals, determine the yield (based on limiting reagent), the percent yield, the melting point, and, if requested by your instructor, determine the IR spectrum. Put your product in a labeled vial and show it to your instructor (if requested).
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| dicyclopentadiene (dimer) | 132.21 | 30 mL | 222 | -1 | 170 | 0.978 | msds | |
| 1,3-cyclopentadiene (monomer) | 66.105 | 5 mL | 60.8 | -97.2 | 40.8 | 0.8021 | msds | |
| Maleic anhydride | 98.06 | 5.0 g | 51.0 | 52.8 | 202 | 1.48 | msds | |
| Ethyl acetate | 88.11 | 20 mL | 205 | -83 | 77 | 0.902 | msds | |
| Ligroin (petroleum ether) | n/a | 20 mL | n/a | 60 | .7 | msds | ||
| endo-bicyclo[2.2.1]hept-5-ene-2,3-dicarboxylic anhydride | 164.16 | 165 | msds | |||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
Answer to question in the text: The cyclopentadiene undergoes dimerization at 25oC to produce dicyclopentadiene, using the Diels-Alder reaction, where one cyclopentadiene molecule is the diene and the other cyclopentadiene molecule is the dienophile.
Melting point of desired produce is either 145oC, 165oC, 185oC. Which one? Well, you need to figure it out, but at least you have a melting point range, meaning that you don't need to worry about checking too vigorously when you are in the 110-120oC range.