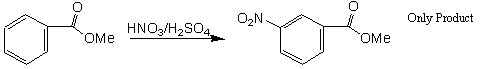
The aromatic ring is exceptionally stable due to resonance and the so-called aromatic stabilization. Few reactions disrupt this thermodynamic stability. Therefore, most of the reactions that involve compounds with the aromatic ring do nothing to change the aromatic nature of that ring. Such is the case in the experiment outlined here. Electrophilic Aromatic Substitution (EAS) is the name given to a reaction that allows an electrophile (a Lewis acid) to replace (substitute for) a H+ on the ring. In essence, a positively charged electrophile substitutes for an outgoing positive electrophile (H+). This is EAS. In the current experiment a nitronium ion (NO2+), prepared by mixing concentrated nitric and sulfuric acids, will get attached to the aromatic ring and the H+ ion released.
The benzene ring is a component of many important natural products and other useful organic compounds. The ability to put substituent groups on a benzene ring, at defined positions relative to each other is a very important factor in synthesizing many new organic compounds. The two main reaction types used for this type of reaction are substitution reactions: Electrophilic Aromatic Substitution (EAS) and Nucleophilic Aromatic Substitution (NAS). The benzene ring itself is electron-rich, which makes NAS difficult, unless there are a number of strong electron-withdrawing substituents on the ring (e.g., aryl halides with multiple fluoride atoms being attached or other aryl halides with nitro groups attached) . EAS, on the other hand, is a very useful method for putting many different substituents on a benzene ring, even if there are other substituents already present. Chapter 12 in Organic Chemistry, by Carey, describes the factors involved in the regioselectivity for EAS reactions using benzene rings which already have substituents on them.
In this experiment you will attach a nitro (—NO2) group onto a benzene ring which already has an ester group attached to it (methyl benzoate). The actual electrophile used in this reaction is the nitronium ion (NO2+), which is generated in situ ("in the reaction mixture") using concentrated nitric acid and concentrated sulfuric acid (see Carey and your lecture notes for the mechanism):
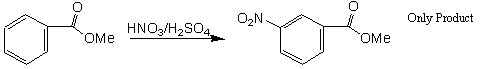
Product Name: Methyl m-nitrobenzoate (Information about naming esters is available online)
Note that only one product is isolated. Why is this the only product? (You should draw resonance structures for the anticipated [meta-substitution] as well as ortho- and para-substituted products.) Why is the ester group electron withdrawing?
The protocol is fairly straightforward. The reaction must be performed in strongly acidic conditions in order for production of the nitronium ion, which, as an electrophile is a Lewis acid. Please be aware of safety considerations, and be careful in all aspects of this reaction.
Safety: Concentrated nitric acid and concentrated sulfuric acid are both strong oxidizers, and highly corrosive — wear gloves while handling them, and avoid breathing their vapors. Methyl benzoate and methyl m-nitrobenzoate are irritants — wear gloves while handling them. Methanol is a flammable liquid, and is toxic — no flames will be allowed in lab, wear gloves while handling it, and avoid breathing its vapors.
To initiate your reaction, prepare the reaction mixture as follows:
After preparing your reaction mixture above, you will need to make a solution containing both concentrated nitric and sulfuric acids. This will be the solution containing the nitronium ion (NO2+) used to attach the nitro group (—NO2) to the benzene ring.
When the addition of the concentrated H2SO4/HNO3 mixture has been completed, let the entire reaction mixture (in the flask) warm to room temperature by letting the flask sit on the bench. Be certain to allow the reaction mixture stand for about 15 minutes after acid addition has been completed in order to allow the reaction to go to completion.
If you do not store your solid product until the next lab period, but decide to do a re-crystallization today, save a small amount of this crude material in a labeled test tube until the next lab period in order to do a melt point determination. Do not seal the tube, since this would prevent evaporation of solvent.
Recrystallize your crude product (collected via vacuum filtration during Day 1) using methanol as your recrystallization solvent.
Use a minimal amount of solvent for this recrystallization, and following the recrystallization protocols from previous experiments (if you think you are not getting a good yield, evaporate some of the methanol), isolated your recrystallized solid.
Record your product yield (both the crude and recrystallized product), melt points, and IR (optional: only if instructed on how to do an IR of a solid sample) of the purified product.
Show your sample to your instructor.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds | |
|---|---|---|---|---|---|---|---|---|---|
| Methyl benzoate | 136.15 | 1.5 mL (1.6338 g) | 12.00 | -12 | 198-199 | 1.094 | 1.5170 | msds | |
| Sulfuric acid, 98% | 98.08 | 3 ml + 1 mL | 3 | 290 | 1.840 | -- | msds | ||
| Nitric acid | 63.0 | 1 mL | -41.6 | 121 | 1.408 | msds | |||
| Methyl m-nitrobenzoate | 181.14 | -- | -- | 78-80 | 279 | -- | --- | msds | |
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |