In this experiment, you will use two important organic separation protocols: extractions and recrystallization. You will start with a mixture of two different organic chemicals (benzoic acid and m-nitroaniline), along with an inorganic salt (NaCl). We will discuss procedures required to separate the benzoic acid from the other components in lab. Once we have the benzoic acid isolated from the other chemicals, we will purify the benzoic acid using recrystallization.
Anyone who has been to Hawaii is probably aware of how sugar (sucrose) is prepared from sugar cane. The sugar cane is grown for a period of time up to two years before it is ready to be harvested. When the crop is ready, the entire field is burned, killing the plants and burning the leaves, leaving only the moist sugar cane stalks. The very dirty stalks are collected, taken to the processing plant and then crushed with large amounts of water being added and the mixture heated. The water dissolves the sugar, leaving the dirt, ash and plant solids behind. The liquid mixture (heterogeneous mixture) contains the dissolved sugar but it is also muddy and dirty. This entire mixture is then allowed to cool, letting the sugar crystallize, but producing still impure sucrose. After several dissolving (and heating) additional recrystallization steps takes place until pure white sucrose is produced. Overall, the process of recrystallization has been used to remove impurities (which remain in the liquid, called the "mother liquor") and produce pure crystalline sucrose.
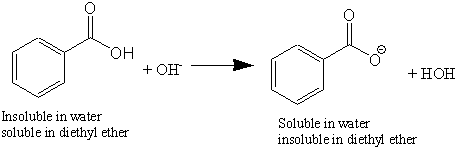
In addition to recrystallization, organic chemists use extractions to purify chemicals from other chemicals that we might not want. Recrystallization is based on the fact that chemicals, when they form crystals, tend to associate with other identical chemicals, hence the ultimate production of pure sucrose crystals which are eventually prepared. For solvent extractions, we rely on the ability to separate one chemical from another chemical utilizing differences in solubility. Most organic chemicals dissolve readily in organic solvents. For example, both of the organic chemicals used in this experiment (benzoic acid and m-nitroaniline), dissolve in ether (actually diethyl ether CH3CH2-O-CH2CH3), and neither of these chemicals dissolve appreciably in water. So, how do we make one of these chemicals partition (i.e., dissolve preferentially) into the aqueous phase? We can chemically alter one of these chemicals to make it "want" to dissolve in water, instead of the ether. We do this with the benzoic acid by neutralizing it when we react it with 10% (~2.5 M) NaOH. This is a simple acid-base reaction. The OH! (hydroxide) ion removes the H+ (proton) ion from the acid, producing the anion of the acid, the benzoate ion.

The benzoate ion is now charged (after the removal of the acidic proton) and prefers to be in the aqueous phase instead of the organic phase. Following this reaction, all the benzoic acid (existing now completely as an ion) will be found in the aqueous phase. Once the aqueous phase is removed from the organic phase you will have purified (extracted) the benzoate ion away from the other organic chemical. You will need to convert the benzoate ion back to benzoic acid by adding 6 M HCl (this step reverses the reaction shown above). Since benzoic acid is almost completely insoluble in water, it will form a precipitate (this is recrystallization). You can then isolate the benzoic acid using vacuum filtration in a Büchner funnel.
Finally, in order to characterize your recrystallized product, you will perform a melt point analysis on the isolated benzoic acid. The melting point of a chemical is a useful physical constant that can be used to verify chemical identity and purity. However, you will need to store your recrystallized benzoic acid in the drying oven until the next lab period because you must have completely dry solids in order to do a melt point analysis.
Obtain about 2 grams (you can acquire 1.8-2.1 grams, which is okay; just be certain to record how much you have to three decimal places) of the chemical mixture containing benzoic acid, m-nitroaniline and NaCl. Add about 5 mL of water to a separatory funnel (make certain the stopcock works properly, is tight, but able to be turned, so that it does not leak). Then, add about 15 mL of diethyl ether to the separatory funnel. You should observe two phases. Even though the liquids are each colorless, the ether, which is less dense than the water, will float on top, much like oil floating on water. You should be able to see the dividing line between the two liquids, called the interface. With the separatory funnel resting in a metal O-ring clamped to a ring stand, or in a cork ring (if the metal O-ring is too large for the separatory funnel), add the dry chemical mixture directly to the liquid contents of the funnel. Use the weighing paper you used to weigh the solid as a folded funnel to pour all the dry chemicals into the separatory funnel. Insert a ground-glass stopper into the top of the separatory funnel, and mix the contents by swirling or shaking the contents. You should be able to observe that most, if not all, of the crystals dissolve and disappear from view. At this time, the NaCl will be dissolved into the water, making an aqueous salt solution. The organic chemicals should have dissolved in the ether (if not all dissolve right now, it is okay, just continue with the protocol), making an organic solution, consisting of the ether (solvent) and the benzoic acid and m-nitroaniline (the two solutes).
|
In order to separate the benzoic acid from the m-nitroaniline, you will need to force one of these chemicals into the aqueous phase, which is the more dense lower layer in the separatory funnel. To accomplish this, you will add about 10 mL of the 10% NaOH solution, to make the lower aqueous phase basic (excess OH! ion; turns red litmus blue), and neutralize the benzoic acid (which had been dissolved in the ether phase) and convert it into the benzoate ion (actually sodium benzoate), which is charged and will now extract into the lower aqueous phase. To effect this transfer, you will stopper the separtory funnel and shake it to thoroughly mix the two phases. Periodically, with the stopper firmly in place (keeping a finger on the stopper at all times), turn the separatory funnel upside down (with the tip near the stopcock pointing upward). Open the stopcock to relieve any pressure (you will probably hear a hiss or the sound of escaping gas). (The reason you generate gas pressure is because the boiling point of ether is about 35oC, which is lower than your body temperature, which provides enough heat in your hands to evaporate some of the ether, creating the gas pressure.)
After you mix the contents of the separatory for a couple minutes, and venting the funnel 3-4 times, you should be ready to separate the aqueous phase from the upper organic phase. Place the separatory funnel in the O-ring clamp (or cork ring) and let the two phases separate completely. When a sharp interface is apparent, remove the ground-glass stopper and then carefully open the stopcock. The lower liquid (a basic solution containing the NaCl, NaOH, and sodium benzoate) will be collected in a 100-mL beaker. Be certain that none of the upper organic phase is allowed to go into the stopcock. Dispose of the organic material into the liquid waste container in the hood.
|
To the collected aqueous phase, which is in the 100-mL beaker, you will add enough 6 M HCl (if you added about 10 mL of 10% NaOH [2.5 M], you will need to add at least 5 mL of the 6 M HCl [you cannot add too much, so, to be safe, add 10 mL of your HCl solution]) to react with the sodium benzoate and convert it back into benzoic acid, which is mostly insoluble in water. You will be able to tell if you have added enough HCl when the solid stays, and does not dissolve when the beaker is swirled or stirred. When you think you have added enough acid, using some blue litmus paper, verify that the liquid is acidic (blue litmus paper turns red, if acidic). When you know that the liquid is acidic (remember that you cannot add too much acid, so a little extra HCl won't be bad), you are ready to collect the recrystallized benzoic acid.
The recrystallized benzoic acid prepared in Part A will be collected by vacuum filtration, dried, and its melting point determined. Depending on the amount of time left in the lab, you may have to store your solid benzoic acid in the drying oven until the next laboratory period. All chemicals store in the drying oven must be labelled appropriately or they may be discarded by stockroom personell.
To collect your solid chemical, set up a vacuum filtration apparatus as described by your instructor. Using a vacuum trap, attach one end of the tubing to one of the vacuum valves, using the rubber tubing attached to the short piece of glass tubing in the stopper. The stopper is then placed in a vacuum trap bottle and the other piece of rubber tubing connected to your 250-mL vacuum (suction) flask. Insert a small Büchner funnel (with rubber stopper) into your flask. Turn on the vacuum. Place a piece of pre-weighed Büchner funnel paper into the top of the funnel and wet their entire filter with water. Pour the entire contents of your beaker containing the benzoic acid into the funnel, with the vacuum on. Use your wash bottle to rinse the remaining solid chemical from the beaker into the funnel. Use a small amount of water to rinse the crystals. Leave the vacuum on for about 5 min to draw air through your chemical and filter paper to assist drying. Remove the filter from the Büchner funnel, and place it in an evaporating dish or watch glass to continue drying in the drying oven, , probably until the next lab period.
After your sample is completely dry (probably during the next lab period, or when the filter paper feels dry), weigh your sample. Because you already know the mass of the filter paper (and perhaps the mass of your evaporating dish), determine the yield of benzoic acid. Using a melt point capillary tube, insert enough chemical to produce about a 1-2 mm height of chemical in the sealed end of the capillary tube after forcing it to the bottom. Too large of an amount of solid will result in a larger melting point range, and should be avoided. Repeat this process using some of the pure benzoic acid provided on the reagent cart. Insert the capillary tubes containing your recrystallized benzoic acid along with the tube containing pure benzoic acid into the melt point apparatus. Turn the temperature control knob to about the 4-5 setting, and monitor for increase in temperature. As the temperature increased to about 100oC, you can turn the unit down a little so that the temperature increase is about 1oC every 10-15 sec. You should make a notation when your solid starts to melt (it turns to liquid) and when all the solid is completely melted. This is your melt temperature range. For example, if it started to melt at 119oC and was fully melted at 123oC, then your melt point range would be: 119-123oC. What is the correct melting point for benzoic acid?
Go To Experiment:
1 2 3
4 5 6
7 8 9
10
Return to Chem102 Experiments Index
Copyright © Donald L. Robertson (Modified: 09/10/2009)