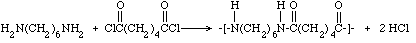Experiment 9
The Formation of the Wonder Polymer: Nylon, an Amide
Objectives
The objective of this experiment is to see how a polymer, nylon is made. This
experiment is referred to as a "Condensation Polymerization Reaction" where
smaller components are combined in a specific order to produce the wonder
polymer nylon. You will create a form of nylon referred to as Nylon 6-6 (both
building-block components contain six carbons. As a contest, your Instructor
might give extra points for the group producing the longest continuous piece of
nylon. Can you beat 79 feet?
Background
The word "nylon" is used to represent synthetic polyamides. The various
nylons are described by a numbering system that indicates the number of carbon
atoms in the monomer chains. Nylons from diamines and dicarboxylic acids are
designated by two numbers, the first representing the diamine and the second the
dicarboxylic acid. Thus nylon 6-10 is formed by the reaction of
hexamethylenediamine and sebacic acid. In this demonstration the acid chloride,
sebacyl (or Sebacoyl) chloride, is used instead of sebacic acid. The equation
is:
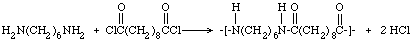
Many diamines and diacids (or diacid chlorides) can be reacted to make other
condensation products that are described by the generic name "nylon." One such
product is an important commercial polyamide, nylon 6-6 (because there are six
carbons in both organic compounds), which can be prepared by substituting
adipoyl chloride for Sebacoyl chloride in the procedure described here. The
equation is:
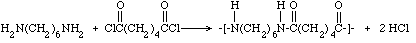
Experimental Procedure
Time: About 30-45 minutes of class time.
Safety Guidelines
- Hexamethylenediamine is irritating to the skin, eyes, and respiratory
system.
- thSodium hydroxide is extremely caustic and can cause severe burns at
high concentration. Contact with the skin and eyes must be prevented.
- Sebacoyl chloride is corrosive and irritating to the skin, eyes, and
respiratory system.
- Hexane is extremely flammable. Hexane vapor can irritate the respiratory
tract and, in high concentrations, be narcotic.
- Wash hands frequently after handling the nylon.
Part I: Synthesis of Nylon 6-6
- Wearing gloves (if you do not have gloves be sure to wash your hands
frequently), pour about 25-30 mL of the hexameylenediamine solution into a
100-125-mL beaker or crystallizing dish. (You could add one drop of
phenolphthalein solution to the beaker prior to the addition of the diamine
solution.)
- Slowly pour the Sebacoyl chloride solution down the side of the beaker to
establish a second layer on top of the diamine solution, taking care to
minimize agitation at the interface. (Tilt the beaker and slowly pour the
Sebacoyl chloride solution down the inside of the beaker.)
- With forceps, grasp the polymer film that forms at the interface of the
two solutions and pull it carefully from the center of the beaker.
- Wind the polymer read on a stirring rod or a small windlass (a piece of
paper towel rolled on itself works fine).
- Wash the polymer thoroughly with water or ethanol before handling.
The phenolphthalein dye, which can be added to the lower (aqueous) phase,
will enhance the visibility of the liquid interface. The upper phase can also be
colored with dyes such as azobenzene, but observation of the polymer film at the
interface is somewhat obscured. Some of the dye will be taken up with the
polymer, but can be removed by washing with water.
Disposal
- Any remaining reactants should be mixed thoroughly to produce nylon. The
solid nylon should be washed before being discarded in a solid waste
container.
- Any remaining liquid should be discarded in a solvent waste container or
should be neutralized with either sodium bisulfate (if basic) or sodium
carbonate (if acidic) and flushed down the drain with water.
Part II: Solubility of amines
During this part of the experiment, you will determine what makes amines
soluble. Most organic chemicals that contain more than about four carbons,
generally are not soluble in water. Many drugs fit into this category. Some of
these drugs, for example, contain the term "hydrochloride" in their names. For
example, morphine might be listed as "morphine hydrochloride" instead of just
"morphine." To produce a compound in the "hydrochloride" form, you smiply add
some hydrochloric acid (HCl) to the compound, and then isolate the resulting
compound.
What is the purpose for is addition of HCl to the compound? There are
actually two purposes. The first is to help make the compound soluble, when it
might not dissolve in water. The second consideration is that adding HCl will
convert the compound into an ionic compound that does not vaporize.
To do the experiment today, you will take two large test tubes containing:
- Add 5 mL of either DI water or 6 M HCl to two different test tubes.
- Add about 0.100 g of 3-nitroaniline (m-nitroaniline) to each of
these test tubes (pour a little of the chemical into a beaker and take the
beaker to your workbench to weigh out your samples).
- Place each of these labelled tubes in a boiling water bath until the
chemical is dissolved. (You may need to mix the tubes to get the chemicals
to fully dissolve in the hot liquid.)
- Remove the test tubes from the water bath and place in your test tube
rack.
After the tubes have cooled, make observations and answer the following
questions.
- Does the chemical dissolve and stay dissolved equally well in both
solvents? /li>
- How can you explan the differences? /li>
- What is the explanation for any differences? /li>
- What application does this have towards pharmaceutical drugs that contain
amino groups?
After you are finished with this part of the experiment, dispose of the
contents from each tube into the liquid waste container and wash your tubes with
soap and water.
Materials and Supplies (some items prepared by stockroom):
- (Prepared by Stockroom) 25-30 ml 0.50 M hexamethylenediamine
(1,6-diaminohexane), H2N(CH2)6NH2,
in 0.5 M sodium hydroxide, NaOH (To prepare: dissolve 3.0 g of H2N(CH2)6NH2
plus 1.0 g NaOH in 50 ml distilled water. Hexamethylenediamine can be
dispensed by placing the reagent bottle in hot water until sufficient solid
has melted and can be decanted. The melting point is 39-40oC.)
- (Prepared by Stockroom) 25-30 ml 0.2 M Sebacoyl chloride,
ClCO(CH2)8COCl, in hexane (To prepare: dissolve 1.5 ml
to 2.0 ml Sebacoyl chloride in 50 ml hexane.) gloves, plastic or rubber
(ones that will not dissolve in hexane)
- 100-125 ml beaker or crystallizing dish
- forceps
- 2 winding rods for collection of fibers (see instructor)
- phenolphthalein (optional)
Go To Experiment:
1 2
3
4 5
6
7 8
9
10
Return to Chem102 Experiments Index
Copyright © Donald L. Robertson (Modified:
11/19/2012)