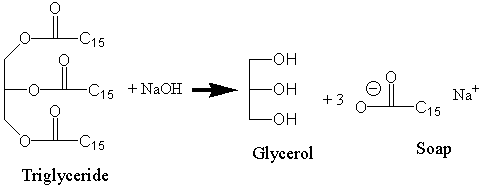
In today's experiment, we will perform a reaction that has been used for millennia: the making of soap. Animal fat and vegetable oils are composed principally of esters of the long chain fatty acids and glycerol (glycerin; 1,2,3-propantriol). Hydrolysis of these triglycerides (triacylglycerides; TAG) in base (e.g., NaOH) yields glycerol (a carbohydrate) and the sodium salts of the fatty acids. Because the fatty acids are ions, they are soluble in low concentrations in water (actually they are soluble because they form micelles), but in high concentration form insoluble aggregates called soap. You will start with a vegetable oil and will use NaOH to hydrolyze these triglycerides. Basic hydrolysis of esters is called saponification. The reaction for this experiment is shown:

The triglycerides most commonly used to make soap commercially are from animal sources, such as tallow, although plant fats from coconut, palm and other vegetable oils can be used. Pure coconut oil yields soap that is very soluble in water because it contains predominately myristic and lauric acids (14- and 12-carbon fatty acids, respectively). Soaps made from animal and other vegetable sources contain more 16- and 18-carbon fatty acids and are generally harder and easy to form into shapes. To soften these harder soaps, coconut oil is often included in the saponification reaction to make the soap softer.
Caution: When you prepare the soap you will need to boil the mixture
until most of the water is evaporated. This may lead to splattering
so be careful that the hot mixture does not splash on you. Follow the
precautions listed below.
Note: If desired the soap for this experiment may be prepared for the lab by the instructor or a specific student group in a larger portion. Ask your instructor whether each group will prepare their own soap, or if soap will be prepared for the entire lab.
Prepare a mixture of 15 mL of 20% (5 M) sodium hydroxide and 10 mL of vegetable oil in a 150-mL flask. Add a stirring bar to the flask, to prevent explosive boiling of the NaOH-oil mixture. Turn the stirring hot plate on at any setting to get it boiling, and then switch to the lowest setting when boiling begins. Turn on the stirrer to let the stirring bar rotate. Boil the mixture, observing the precautions listed above. Carefully control the heating, but you should heat the mixture high enough to maintain a constantly boiling mixture. The saponification is complete if a wax-like solid begins to form that on further cooling becomes hard and somewhat brittle. On the other hand, if the mixture cools to a syrupy liquid, saponification is not complete, and heating and stirring must be resumed. It might be advisable to add more (5 mL) 20% NaOH and boil the mixture until its water is expelled. Saponification should be complete by 30-45 min (but it may take only 15-20 min).
While the mixture is heating, prepare a concentrated salt solution by dissolving 50 g of NaCl in 150 mL of distilled water in a 400-mL beaker. (Prepare this solution immediately so that it is ready when your soap is ready.) When the saponification reaction is complete, remove the flask from the heat source using HotHandsTM to hold the hot flask. Pour the reaction mixture quickly into the saturated salt solution (you may have to scrape the solid into the NaCl solution using a scoopula). Stir the mixture thoroughly for several minutes; then, collect the precipitated soap on a Büchner funnel. Wash the soap twice with 10 mL of ice-cold distilled water (cool the water with ice, but don't add the ice to the distilled water). After you have collected and washed the soap, continue to draw air through the soap for several minutes to help dry it. Save the soap for use in the evaluation section.
Evaluation of Soap
Dissolve about 1 g of your soap in 50-60 mL of boiling distilled water (this is the Soap Solution). Add about 1 g of detergent (provided by the lab) into about 50-60 mL of distilled water (use hot water if it is solid detergent, or room temperature water, if liquid); this solution will be the Detergent Solution. If either the soap or detergent solution is cloudy, this solution must be filtered prior to being used in the procedures outline below (rinse your filter flask and filter your cloudy solution through a small Büchner funnel, collecting the filtrate for experimental purposes).
Experiment 1: Emulsification (dissolving) of Oils
Place 4 drops of mineral oil in each of three tubes. Add 5 mL of distilled water to one tube, 5 mL of your soap solution to another, and 5 mL of your detergent in the last tube. Shake the tubes briefly and observe how well the oil is emulsified in each. Record your observations in the table below.
| Tube #1: Water | Tube #2: Soap Solution | Tuber #3: Detergent Solution |
|
|
|
|
Experiment 2: Reaction with metal ions
Although the sodium and potassium salts of common soaps are soluble in water, the metal cations Mg2+, Ca2+, and Fe3+, which are typical components of "hard" water, form insoluble complexes. The insoluble complexes form a scum on the top of the water, and lead to the "ring around the tub" problem. Most detergents, however, do not respond in a similar manner, and can be used in both "hard" and "soft" water.
Place 5 mL of your soap solution in each of three test tubes. Add to each tube 2 mL of a 1% solution of CaCl2, MgCl2, and FeCl3 and note whether a precipitate forms. Shake each tube and observe what happens. Repeat using the detergent solutions.
| Tube #1: Soap plus Mg2+ | Tube #2: Soap plus Ca2+ | Tube #3: Soap plus Fe3+ |
|
|
|
|
| Tube #1: Detergent plus Mg2+ | Tube #2: Detergent plus Ca2+ | Tube #3: Detergent plus Fe3+ |
|
|
|
|
Experiment 3: Is soap basic or acidic?
Since soap is a sodium salt of the fatty acids, you will determine if the acid form of these fatty acids (in contrast to their anionic forms) is soluble in water. First, add a drop of phenolphthalein (a chemical which turns pink in basic solution, with pH greater than 7.0, but which is colorless in acidic solutions) to a 5-mL sample of your soap and of your detergent. Note any observations. Remember that a pink color indicates that your solution is basic (with a pH greater than 7.0).
(You can use the tubes from above, with the phenolphthalein in them, for the next part of the experiment.)
Reaction with acid: Second, test a 5-mL sample of your soap solution and a sample of your detergent solution by adding 5 mL dilute (3 M) HCl. (If the color of the solution is still pink, due to the phenolphthalein, then keep adding acid until the pink color disappears.) When the pink color is gone, the solution will be acidic. Note your observations (i.e., is the contents of the tube clear? is it cloudy? does it bubble?).
| Tube #1: Soap plus phenolphthalein | Tube #2: Detergent plus phenolphthalein |
|
|
|
| Tube #1: Soap plus HCl (pink color is gone) | Tube #2: Detergent plus HCl (pink color is gone) |
|
|
|
If there will be a single, lab-scale preparation of soap for the lab, your instructor, or designated group, should follow the procedure below:
Prepare a mixture of 75 mL of 20% (5 M) sodium hydroxide and 50 mL of vegetable oil in a 500-mL Erlenmeyer flask. Add a large stirring bar to the flask, to prevent explosive boiling of the NaOH-oil mixture. Turn on the stirrer to let the stirring bar rotate. Turn the stirring hot plate on at any setting to get it boiling, and then switch to the lowest setting possible when boiling begins. Boil the mixture, observing the precautions listed above. Carefully control the heating, but you should heat the mixture high enough to maintain a constantly boiling mixture. The saponification reaction is complete when a wax-like solid begins to form, that upon further cooling becomes hard and somewhat brittle (like soap). On the other hand, if the mixture cools to a syrupy liquid, saponification is not complete (oil is remaining). Continue heating and stirring your reaction, and it might be advisable to add more (15-20 mL) 20% NaOH to the boiling mixture. Saponification should be complete by 30-45 min (but it may take only 15-20 min).
While the mixture is heating, prepare a concentrated salt solution by dissolving 100 g of NaCl in 250 mL of distilled (DI) water in a 600-mL beaker. (Prepare this solution immediately so that it is ready when your soap is ready.) When the saponification reaction is complete, remove the flask from the heat source using HotHandsTM to hold the hot flask. Pour the reaction mixture quickly into the saturated salt solution (you may have to scrape the solid into the NaCl solution using a scoopula). Stir the mixture thoroughly for several minutes; then, collect the precipitated soap on a Büchner funnel. Wash the soap (in the funnel) twice with 50-100 mL of ice-cold distilled water (cool the water with ice, but don't add the ice to the distilled water since the ice is made from tap water). After you have collected and washed the soap, continue to draw air through the soap for several minutes to help dry it. Save the soap for use in the evaluation section.
Dispense small amounts of soap to each student group for use in their experiments.
Go To Experiment:
1 2 3
4 5 6
7 8 9
10
Copyright © Donald L.
Robertson (Modified:
09/10/2009)
Return to Chem102 Experiments Index