In this experiment, you will purify isopropanol (2-propanol) by simple distillation. This technique shows you how to purify volatile organic compounds by distillation. Non-volatile chemicals (such as the dye used in this experiment) does not go into the gas phase, and is effectively separated from the volatile compound. This procedure is good for the purification of most volatile compounds from compounds that are not volatile, but not very successful for mixtures of compounds which are all volatile (an alcohol and water mixture). On the other hand, you might want to keep the non-volatile compound, such as for the isolation of salt by evaporation.
The most common method for separating and purifying volatile liquids is distillation, which makes use of the specific boiling points of the liquid components in the mixture. When there is only one volatile compound, or when one of the liquids has a boiling point well below the others, a simple distillation is used. However, if there are two or more liquid components, which have boiling points near each other, a fractional distillation must be used. We will separate isopropanol from a dye, which is not volatile.
Using distillation, we will separate 2-propanol (isopropyl alcohol or isopropanol) from a non-volatile dye. While performing the distillation, you will monitor the temperature that the volatile liquid has, in the gas phase, using a thermometer. This temperature should remain constant, and actually represents the boiling point of this chemical. You should report the amount of isopropanol you isolate and its boiling point.
|
Safety: 2-propanol is a highly flammable liquid and a severe eye irritant -- no flames will be allowed in lab while it is in use. As for every experiment, goggles must be worn, even though you may not actually be working the chemicals, if there is anyone using 2-propanol in the lab.
Your instructor will explain how to set up a simple distillation apparatus using some of the glassware available to you in the organic chemistry kits. You will use a 100-mL round bottom flask as the distillation pot, and a 50-mL round bottom flask as the receiver. As a standard rule, anytime you are boiling an organic compound, you will always include ~ 4-5 (not a handful!) boiling stones to keep the solution boiling smoothly, and to prevent "bumping" of the liquid!
Obtain about 50 mL of the "impure" isopropanol (this sample of isopropanol has had a small amount of a soluble, non-volatile dye added to it as an impurity). Add the isopropanol to the distillation pot, add a few (~ 4-5) boiling stones, and begin the distillation (remember to turn on the cooling water before you turn on the heat). [Please have your instructor check your distillation setup before you turn on the heat or water, to make certain everything is setup correctly!] Start recording the temperature for every minute of the distillation. You can stop the distillation when you have collected about 25 mL of isopropanol (you can collect your sample in a graduate cylinder), but do not let your distillation pot go dry. You can plot your data using graph paper, or you can plot your data using a computer program.
After you have collected your distillate (the distilled liquid), and you have stopped the distillation by turning off the heating mantle and lowering the mantle from the distillation pot, let the entire setup cool for about 10 min.
Analyze your collected isopropanol by doing the following:
After you determine the density and the refractive index, dispose of your liquid, and any liquid remaining in the distillation pot, in the liquid waste cpntainer. Be make certain that no boiling stones are transferred into the liquid waste. Put the boiling stones in the solid waste container.
1. What is the apparent boiling point of the isopropanol, as determined from your experiment? What is the actual real boiling point of isopropanol (from a reference manual or internet)?
2. Make a graph of temperature (Y-axis) verses time (X-axis) for your distillation. How can you use this information to determine the boiling point?
3. What was the refractive index of your isopropanol? What is the actual refractive index of isopropanol (from a reference source)?
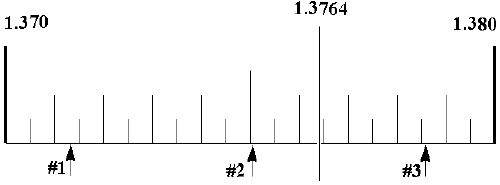
Refractive index is a measure of how light "bends" or "refracts" when it passes through a substance. The refractive index for a given chemical is one of its physical properties. Your instructor will show you how to use the refractometer. After determining the refractive index, you will need to read its value from the scale in the machine. The figure below is illustrative of how to determine the correct value. After you align the "horizon" with the cross-hairs, you must obtain the refractive index value. Part of this scale is shown below:

As an exercise, please give the values for the refractive index for each of the arrows (to 4 decimal places):
#1 ______________________ #2 ______________________ #3_______________________
Refractive index must include four decimal places. For example, if
the vertical marking is in the location shown above, the experimental value
would be 1.3764.
Go To Experiment:
1 2 3
4 5 6
7 8 9
10
Copyright © Donald L.
Robertson (Modified:
09/10/2009)
Return to Chem102 Experiments Index