Aspirin, the Wonder Drug, is an important chemical world-wide. The method described before is that followed by most manufacturers to make this chemical. This experiment teaches the concept of ester formation using a very reactive acid derivative (acetic anhydride) with an appropriate alcohol (salicylic acid). Chemical purification using vacuum filtration is used. Once the chemical is dried, its yield (theoretical and actual), percent yield and melt temp can be determine. This experiment is a great way to relieve headaches!
Aspirin is a trade name for acetylsalicylic acid, a common analgesic. Acetylsalicylic acid is an acetic acid ester derivative of salicylic acid. The earliest known uses of the drug can be traced back to the Greek physician Hippocrates in the fifth century B.C. He used powder extracted from the bark of willows to treat pain and reduce fever. Salicin, the parent of the salicylate drug family, was successfully isolated in 1829 from willow bark. Sodium salicylate, a predecessor to aspirin, was developed along with salicylic acid in 1875 as a pain reliever. Sodium salicylate was not often popular though, as it has a habit of irritating the stomach. However, in 1897, a man named Felix Hoffman changed the face of medicine forever. Hoffman was a German chemist working for Bayer. He had been using the common pain reliever of the time, sodium salicylate, to treat his father's arthritis. The sodium salicylate caused his father the same stomach trouble it caused other people, so Felix decided to try and concoct a less acidic formula. His work led to the synthesization of acetylsalicylic acid, or ASA. This soon became the pain killer of choice for physicians around the globe. Scientists never really understood the inner workings of the drug however. It wasn't until the 1970's, when British pharmacologist John Vane, Ph.D. began work on aspirin that people began to understand how aspirin really works. Vane and his colleagues found that aspirin inhibited the release of a hormone like substance called prostaglandin. This chemical regulates certain body functions, such as blood vessel elasticity and changing the functions of blood platelets. Thus can aspirin affect blood clotting and ease inflammation.
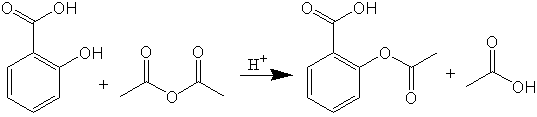
The reaction for synthesis of acetylsalicylic acid is shown in the following figure.

In previous experiments, we used Fischer esterification reaction to produce some esters that we detected by odor. The current experiment uses, instead of glacial acetic acid (concentrated acetic acid), another carboxylic acid derivative, acetic anhydride for ester formation. The advantage of using acetic anhydride is that you do not produce water which can be used for hydrolysis of the newly formed ester. Concentrated phosphoric acid will be used to keep everything in the acidified, protonated state. Acetic anhydride is the preferred acid derivative to synthesize aspirin commercially because the acetic acid produced in this reaction can be used again, by converting it back into acetic anhydride.
In a 125-mL erlenmeyer flask, add 2 g salicylic acid (put the flask on the balance, and zero it). In the hood, carefully add 5 mL of acetic anhydride (severe irritant, handle carefully) to the flask. Slowly add about 10 drops of 85% phosphoric acid (H3PO4). Stir the mixture with a stirring rod. Place the flask and its contents in a boiling water bath and stir untill all the solid dissolves. Remove the flask from the hot water and let it cool. Working in the hood, add 20 drops of water to the cooled mixture. (Avoid breathing any of the vapors, which contain acetic acid, and are irritating).
When the reaction is complete, add 50 mL of cold water to the reaction mixture. Cool the mixture by placing the flask in an ice bath for 10 minutes. Stir occasionally to keep mixture in the reactive state. Crystals of aspirin should form. If no crystals form, gently scratch the sides of the flask with the stirring rod.
Collect the aspirin crystals using a Buchner funnel. Set up a Buchner funnel as described by your instructor, or in a manner performed previously. Add a pre-weighed filter into the funnel and wet it so that it seals completely when vacuum is applied. Pour the aspirin crystals into the funnel and collect the white solid. Add a little cold water to the flask and make certain all the crystals are transferred to the funnel. After you have washed the crystals, keep the vacuum on for about 5 min to help dry the crystals. Turn off the vacuum, and transfer the filter paper and crystals to a pre-weighed beaker. Let this material sit at room temperature until the next lab period when you will determine your aspirin recovery.
| Compound | MW | Amount Needed | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Salicylic acid | 138.12 | ~2.0 g | 14.5 | 159 | 211 | 1.44 | --- | view |
| Acetyl salicylic acid (aspirin) | 180.16 | --- | 135 | 140 | 1.35 | --- | view | |
| Acetic anhydride | 102.09 | 5.0 ml | -73.1 | 139.9 | 1.08 | 1.389 | view | |
| Compound | g/mol | grams or mL | 10-3 M | oC | oC | g/mL | ηD | msds |
Go To Experiment:
1 2
3
4 5
6
7 8
9
10
Return to Chem102 Experiments Index
Copyright © Donald L. Robertson (Modified: 11/19/2012)