Experiment 5
Synthesis of
Tris(2,4-pentanedionato)iron(III) & Tris(ethylenediamine) nickel(II)
chloride
Outline of
Experiment:
- Day 1
- Prepare Tris(2,
4-pentanedionato)iron(III)
- Day 2
- Recrystalize the
Tris(2, 4-pentanedionato)iron(III) (optional, at instructor
discretion)
- Prepare
Tris(ethylenediamine) nickel(II) chloride
Part A:
Stoichiometry and Synthesis of Tris(2, 4-pentanedionato)iron(III)
For this
experiment, you will synthesize a new compound, or set of compounds, from the
starting reactants. Use only the quantities of material listed in the
handout. Determine the limiting reactant which will be used to determine
your theoretical yield. Be certain that you can identify the correct
limiting reactant, and disregard compounds that are used as solvent and which
are not present in the product.
Hydrates are
compounds which contain water molecules as an integral part of their
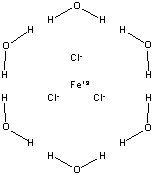
composition. The molar mass of the compound we will be using, Iron(III)
chloride hexahydrate (FeCl3·6H2O), is 270.2952 g/mol,
which includes the FeCl3 (162.204 g/mol, which is the molar mass of
anhydrous FeCl3) but also the six (6) moles of water (total mass is
108.0912 g/mol compound) which are an integral part of the complex, shown
below.
|
FeCl3•6H2O Molar Mass is 270.2952 g/mol |
|
You should
calculate the molar mass of the compounds used in this experiment yourself to
verify these values. When you calculate the number of moles of a compound
which exists as a hydrate, you must include the total molar mass, including the
water, not just the anhydrous (without water) part.
For this
experiment, the Iron(III) chloride hexahydrate will be reacted with
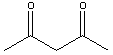
2,4-pentanedione, which is shown below.
|
2,4-pentanedione (C5H8O2) Molar Mass is 100.117 g/mol |
|
Follow the
published experimental procedures. When requested to obtain about
1.3 g FeCl3·6H2O, you should weigh out about this
amount, but it does not need to be exactly 1.300 g (about means close,
but necessarily exactly this amount, meaning that 1.250 g is okay, as well as
1.345 g). Record your mass to three (3) decimal places. The
reactants for this experiment are the FeCl3·6H2O and
2,4-pentanedione (2.00 mL; density is 0.957 g/mL). The other chemicals
included in the reaction are either solvent (methanol) or buffer (sodium
acetate), and are not included in limiting reactant calculations. When
the two reactants are mixed, there will be an intense color change, turning
blood red. In fact, this reaction is similar to the interaction of iron
with oxygen in blood, which, of course, is blood red. Our new
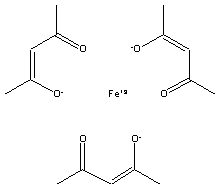
compound is Tris(2,4-pentanedionato)iron(III), which is shown below.
|
Tris(2,4-pentanedionato)iron(III) Molar Mass is 353.1723 g/mol |
|
The reaction is:
|
FeCl3•6H2O (aq) + 3 C5H8O2 (MeOH) + 3 NaC2H3O2•3H2O
(aq) → |
|
|
Reactants:
|
Products:
|
After you have made
your product, and collected it via vacuum filtration, you should dessicate
(dry) your solid until the next lab period for quantitation (a
recrystallization may be performed on the second day, if your instructor
requests it). It is helpful to pre-weigh the dry filter paper prior to
doing the filtration, as well as the dry plastic container, so that when you
have dried your sample, to determine the amount of product, you will weight the
plastic container, with the solid product and filter paper, and subtract the
mass of the container and filter paper. After you obtain a mass for the
crystallized material, you should calculate a percent yield based on the theoretical
yield determined from the mass (moles) of the limiting reactant.
Label your
container by putting your Name and "DLR110-Exp5A"
on the label (use labelling tape available on the front bench). Be sure
to mass the container and transfer all of the solid product, along with the
pre-weighed filter paper, into the container. Do not put the lid on the
container, because the lid, if attached, will prevent evaporation and and your
sample will not dry. Place the sample (container, filter paper, and
reaction product) in the dessicator (drying chamber) to dry until the next lab
period.
At the beginning of
the next lab period, retrieve your container from the dessicator and weigh the
entire contents. You can calculate the actual yield by subtracting the mass of
the dry container and the dry filter paper from the total mass just determined.
You will compare the mass of this crude product, with the mass you
recover as recrystallized material (if required to do a recrystallization)
using the recrystallization procedure described below, during the second day of
this lab. A recrystallization step will yield a purer product, but will affect
your yield, especially if you do not follow the instructions carefully.
After this recrystallization step, you will dessicate your product until
the next lab period and quantitate your yield at that time.
|
Lab Protocol for Day 1 |
|
Optional
Recrystallization (from original protocol written by Mark Yeager) - perform
only if requested by your instructor.
To understand
the purpose of recrystallization, see the article about the recrystallization
of sugar at the end of this experiment.
Gently scrape the
solid product off of the filter paper into a 150-mL beaker. Try not to
scrape bits of paper along with the product, as these insoluble particles will
be collected by filtration and contribute to the mass of your recovered
product. Dispose of the filter paper in the "Filter Paper"
waste container (or the container marked as solid waste). Dissolve the
solid product in the beaker by adding about 1-2 mL of methanol, and stirring
with a glass stirring rod. If the solid does not all dissolve, add
another 1-2 mL of methanol (you may have to keep adding methanol and stirring
to get all the solid to dissolve, but be sure to add no more than 2 mL of
methanol at a time. It may take as much as 15-20 mL of methanol to
dissolve all of the product.
Add 25 mL of water
to the product/methanol solution. The product will recrystalize from the
solution, since it is soluble in methanol but not in water. As it
recrystallizes, a lot of the impurities that were present in the original
product stay dissolved in the methanol/water solvent. However, some of
the product will also stay in solution, which means that some yield is
sacrificed in order to get a pure product.
Suction filter the
recrystallization mixture. Label your container by putting your Name
and "DLR110-Exp5A" on the label (use labelling tape
available on the front bench). Mass the container and transfer the solid
product, along with the pre-weighed filter paper, into the container. Do
not put the lid on the container, because the lid, if attached, will prevent
evaporation and and your sample will not dry. Place the sample
(container, filter paper, and reaction product) in the dessicator (drying
chamber) to dry until the next lab period. At the beginning of the next
lab period, retrieve your container from the dessicator and weigh the entire
contents. You can calculate the actual yield by subtracting the mass of the dry
container and the dry filter paper from the total mass just determined.
Percent yield is calculated using the formula at the end of this protocol.
|
Lab Protocol for Day 2
(continuation of Part A) |
|
Part B:
Synthesis of tris(ethylenediamine) nickel(II) chloride
You will prepare
tris(ethylenediamine) nickel(II) chloride in this part of the experiment.
This compound is colored, but not red like the experiment in Part A.
Like the experiment in Part A, however, you must do a limiting reactant
determination to find out which of the starting reagents (which appear in the
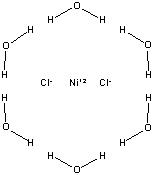
product) is limiting. The reactants for Part B are shown below.
|
NiCl2•6H2O Molar Mass is 237.6906 g/mol |
|
|
Ethylenediamine (C2H8N2) Molar Mass is 60.0986 g/mol |
|
|
Tris(ethylenediamine) nickel(II) chloride Molar Mass is 309.7 g/mol |
Product |
Follow the protocol
shown below, which is not included in your experimental packet. Be sure
to determine the limiting reactant, theoretical yield (based on the limiting
reactant), and percent yield. Answer all of the questions at the end of
this section in your lab notebook.
Synthesis of
tris(ethylenediamine) nickel(II) chloride (Protocol by Mark Yeager; copyright by
Mark Yeager)
The equation for
the synthetic reaction you will carry out is:
NiCl2•6H2O
(aq) + 3 C2H8N2 (aq) → [Ni(C2H8N2)3]Cl2
(s) + 6H2O (l)
The procedure for
making [Ni(C2H8N2)3]Cl2
(tris(ethylenediamine)nickel(II) chloride) is given below. It will be up
to each group to determine what measurements and calculations must be made to
determine the limiting reagent, the theoretical yield and percent yield of the
solid product, and, in the written report, to account for the difference
between theoretical and percent yield (lost product, unreacted starting
material, etc.). Note that the ethylenediamine is provided as an aqueous
solution (C2H8N2 (aq)), not as a pure
compound. You must take this into account in your limiting reactant
calculations.
Safety: In this part of the
experiment you will be using acetone, which is flammable, so there will be no
flames in the lab during this experiment. Ethylenediamine is very corrosive,
and it will be stored in the fume hood to reduce the hazard of any spills. You
should wear gloves when handling this solution, since it is corrosive.
Make sure all
glassware is very clean -- any residue of sodium acetate from Part A will
interfere with this reaction.
(Use the
quantities and procedure shown below, or follow the instructions included in
the published protocols, if desired.)
Measure about 2 to
2.5 grams of nickel(II) chloride hexahydrate, and put it in a 150-mL beaker.
Dissolve it in as little DI water as possible (5 mL maximum). Use a
glass-stirring rod to mix the solution. Slowly add 10.00 mL (carefully
measured) of 25.0% (m/m; mass percent) ethylenediamine-water solution (density
of the solution is 0.950 g/mL), again using the stirring rod to mix. Add about
25 mL of acetone (extremely flammable) to the reaction mixture, in 5
increments of about 5 mL each (thoroughly mix after each 5-mL addition).
Continue stirring until precipitation begins.
Cool the beaker on
ice for about 10-15 minutes to maximize precipitation (if the beaker is warm to
the touch after the addition of acetone, wait for it to cool to near room
temperature before putting it in the ice bath).
|
Suction filter the
product mixture, collecting the solid on the filter paper. If any particles of
product remain in the reaction container, use a rubber policeman to scrape them
into the funnel. If this is not helpful, use a small amount of the filtrate (do
not use water!) to wash the rest of the product into the funnel.
After the liquid
has been drawn through the filter paper, carefully break up the solid with a
spatula, making sure that you don't tear the filter paper. Dry the crystals by
leaving the vacuum on for about 5 minutes.
Remove the funnel
from the flask. Carefully transfer the product from the filter paper to a
pre-massed vial labeled with the product name and the names of the people in
your group. Put the vial in the desiccator. Use the next lab period to
determine the mass of the dry product. Be sure to dispose of all waters in the
proper containers.
Simply dessicate
all of your product until the next lab period and quantitate your yield at that
time. Label your container by putting your Name and "DLR110-Exp5B"
on the label. Do not put a lid on the container, because the lid will
prevent evaporation and and your sample will not dry.
|
Lab Protocol for Day 2
(Part B) |
|
Results
As is always the
case, your Results section must include the important observations and data
from the Experimental section, and any calculations you perform. In this case,
be sure to include those observations that confirm that the product you collect
was the compound you were trying to synthesize. You should also include
calculations to show how you determine which reactant is the limiting reactant,
and the theoretical yield and the percent yield of the products.
Discussion
Specific points
that you should address in this section (in addition to the usual discussion
and explanations) are:
- How do you know that a chemical reaction took
place?
- How do you know that the compound you collected
as a product is the compound you were trying to synthesize?
- How did you determine which reactant was the
limiting reactant?
- What was your percent yield, and does this
reflect a good yield of product?
- What specific observations show where in the
procedure you lost some of the product?
Recrystallization (a description)
Anyone who has been to Hawaii is probably aware of
how sugar (sucrose) is prepared from sugar cane. The sugar cane is grown
for up to two years before it is ready to be harvested. When the crop is
ready, the entire field is burned, killing the plants and burning the leaves, leaving
only the sugar cane stalks. The very dirty stalks are collected, taken to
the processing plant and then crushed with large amounts of water being added.
The water dissolves the sugar, leaving the dirt, ash and plant solids
behind. The liquid mixture (a heterogeneous mixture), which contains the
dissolved sugar, but also is still muddy and dirty, is allowed to crystallize,
producing still impure sucrose. After several dissolving and
recrystallization steps, pure, white sucrose is produced. Overall, the
process of recrystallization has been used to remove impurities (which remain
in the liquid, called "mother liquor") and produce pure crystalline
sucrose.
|
Required Chemicals and
Supplies |
|
|
Day 1 |
Day 2 |
|
|