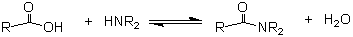
Carboxylic acid derivatives constitute an important class of organic compounds, both industrially and biologically. In this experiment you will synthesize N,N-diethyl-m-toluamide (DEET), which is a compound that is important commercially because of its biological activity -- it is an insect repellent, and is the active ingredient in the"Off" and "Jungle Juice" brands.
Amides appear to be the product of a condensation between a carboxylic acid and an amine:
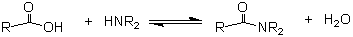
In practice, however, this reaction generally produces only the ammonium salt of the carboxylic acid, due to the acid-base neutralization reaction which takes place:
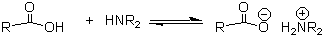
The best sythetic route from a carboxylic acid to an amide (and the one we will use) is to first convert the acid to the acid chloride, and react the acid chloride with the amine. Note, that since HCl is produced, the reaction should be performed in the hood to avoid production of HCl gas in the room.
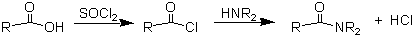
You will use m-toluic acid as the starting reagent.
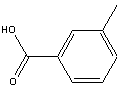 |
Synonyms m-methylbenzoic acid; m-toluylic acid; m-Methylbenzoate; m-methylbenzoic acid |
The reaction is catalysed by dimethylformamide (DMF), which reacts with oxalyl chloride in the first step to give the iminium intermediate
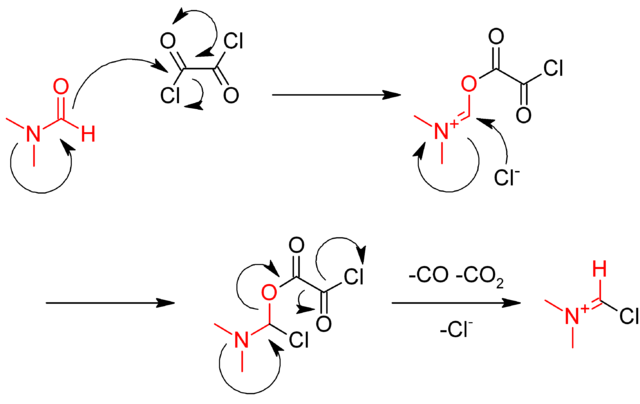
The iminium intermediate reacts with the carboxylic acid, abstracting an oxide, and regenerating the DMF catalyst.
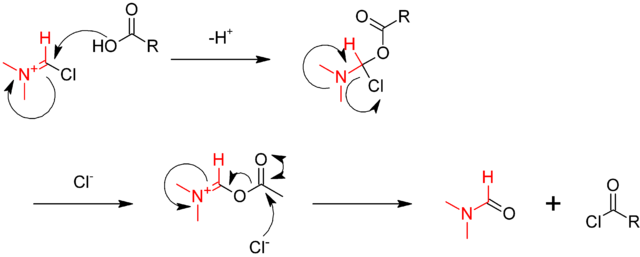
The reaction scheme shown here is usually successful in performing an amide synthesis.
Safety considerations:
Combine the following chemicals in 50- or 100-mL round-bottom flask.
Swirl to thoroughly mix reagents (solid will not totally dissolve until heated).
Reflux the mixture gently for 30 minutes in the hood, since HCl(g) is produced. Cool the mixture to room temperature using an ice bath. Stopper the flask and leave it in the fume hood until you need it later (this mixture now contains reactive m-toluoyl chloride, which is an acyl chloride).
While the reflux is proceeding prepare an Erlenmeyer flask containing the following chemicals (follow the addition instructions indicated):
Slowly add the diethylamine hydrochloride to the aqueous NaOH, with swirling. Once all the diethylamine hydrochloride has been added, and dissolved, add all of the sodium lauryl sulfate.
Transfer the m-toluyl chloride prepared in the first reaction to a separatory funnel, which will be used for dropwise addition of the acyl chlorde to the flask. After each drop or two, swirl the flask to mix its contents. Once the addition is complete, continue swirling the flask for another 5 minutes to ensure complete mixing of reactants.
STOP: You should stopper the flask (using a cork) and seal it with parafilm. Place your properly-labeled reaction mixture on the cart for storage until the next lab period.
Extract the reaction mixture in a Separatory Funnel using two 25-mL portions of ether1 (which phase do you save?). There might appear to be three phases in the Separatory Funnel, but it is the ether phase (top layer) that you want to save. So, remove the lower phase(s) into a beaker. Then, remove all the upper phase to another beaker and save to combine with the second ether wash. Pour the lower phase liquid back into the Separatory Funnel, add your second 25-mL portion of ether and repeat the extraction, collecting only the upper ether phase.
Wash the two combined ether extracts (from above) at least twice using about 1/3 volume saturated NaCl (aq) for each wash. Dry the ether phase using 3-5 grams of anhydrous granular Na2SO4 (avoid using anhydrous MgSO4, which is a fine powder and difficult to remove). You can determine if all the water is removed if the liquid is completely clear (not necessarily colorless). If the liquid remains cloudy, you might try adding a little more Na2SO4 to your flask.
In order to purify your amide product, you will need to evaporate the ether in the hood using a hot water bath on a hot plate (ether boils at 35oC). Place your flask/beaker with your chemical (containing diether ether) in the larger beaker containing water, and let the ether boil off. You should continue to heat until the rapid boiling stops. Be sure that you do not heat your sample directly on a heating plate, as too high of a temperature will be produced causing your product (in addition to the diether ether) to evaporate, since your amide boils at 111oC.
Determine yield, percent yield (what is your limiting reagent?), refractive index (ηD) and take the IR spectrum (your instructor will show you how to apply a very small amount of liquid for the IR analysis). If requested, give the rest of the product to the instructor in a labeled vial (because he may want to make his own insect repellent), otherwise, dispose of your chemical in the liquid waste container.
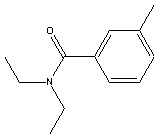 |
Synonyms N,N-diethyl-m-toluamide N,N-diethyl-3-methylbenzamide |
The N,N-diethyl-m-toluamide is the principle reagent in the insect repellent commonly referred to as DEET.
| Compound | MW (g/mol) | Amount Needed | mmol | mp (oC) | bp (oC) | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| m-toluic acid | 136.15 | 3.4 g | 25 | 110 | 263 | 1.054 g/mL | msds | |
| thionyl chloride | 118.9654 | 2.2 mL (use pipettor) | 30 | -104 | 76 | 1.631 g/mL | msds | |
| oxalyl chloride | 126.93 | 3.0 mL (4.365 g) | 34 | -12 | 63-64 | 1.455 | msds | |
| N,N-dimethylformamide | 73.09 | 1 drop | --- | -61 | 153 | 0.949 | msds | |
| 3 M NaOH | 30 ml | 90 | msds | |||||
| diethylamine HCl | 109.5985 | 2.2 g | 20 | 227 - 230 | 320 - 330 | msds | ||
| sodium lauryl sulfate | 288.37687 | 1.0 g | 204 - 207 | msds | ||||
| Diethyl ether, dry | 74.12 | 50 mL | 34.5oC | 0.708 g/mL | 1.3530 | msds | ||
| N,N-diethyl-m-toluamide | 191.2724 | 111 | 0.992 g/mL | 1.5212 | msds | |||
| Saturated NaCl | 58.45 | ~20 mL | msds | |||||
| Na2SO4 (anhydrous) | 142.03714 | ~3-5 g | 884 | msds | ||||
| Compound | MW (g/mol) | Amount Needed | mmol | mp (oC) | bp (oC) | Density | ηD | msds |
1Extractions are performed sequentially. For this experiment, the aqueous reaction mixture, is washed twice with ether. The less dense ether layer, after the first extraction, contains the dissolved organic product. Collect the aqueous layer into a beaker. Transfer the ether layer to a flask. Add the aqueous material back into the separatory funnel and wash again with the second 25-mL ether addition. Discard the aqueous layer. Combine the ether washes in the separatory funnel. Add about 1/3 volume of saturated NaCl solution to the ether phases. Extract at least twice with saturated NaCl. Collect the ether phase. Add anhydrous Na2SO4 to dehydrate the ether (the liquid will become clear).
Go To Experiment: ChemDraw 1 2
3 4
5
6 7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright © Donald L. Robertson (Date last modified: 04/06/2009)