Many naturally occurring chemicals can be used isolated and used to make other compounds. Methyl salicylate is a naturally occurring compound that we will use to produce salicylic acid, which will then be used to make the wonder drug aspirin (acetylsalicylic acid). This is not the source of salicylic acid in the industrial synthesis of aspirin, but it is, nevertheless, a good one for laboratory use. Two different chemical techniques will be employed. First is hydrolysis, which is the breaking of a bond with water. Esters are quite easily hydrolyzed into their two starting components, an acid and an alcohol. Then, we perform an ester synthesis for the formation of aspirin. Equilibrium favors hydrolysis, but using acetic anhydride as the acid source prevents this backward reaction, since water is not produced during ester formation. Both salicylic acid and aspirin are easily isolated.
Methyl salicylate (an ester) can be hydrolyzed to produce salicylic acid. The two different functional groups on the aromatic ring are utilized in this lab. First, the free carboxylic acid group will be produced when we hydrolyze the methyl salicylate. Methanol is the alcohol which is released by hydrolysis. Second, the hydroxyl group on salicylic acid will be used in ester formation to produce aspirin. When we performed an ester synthesis previously, we took special precautions to prevent water from being produced (we included a dessicant in the reaction mixture and had a drying tube affixed on top of the reflux column). Acetic anhydride will be used in this experiment so that when ester is formed, water is not produced. As a result, the thermodynamic equilibrium will now be for the formation of an ester instead of its hydrolysis.
Before proceeding with the experiment you should review several items. First, you should be familiar with the ester functional group. Esters are formed when an acid and an alcohol combine. You should be able to quickly identify an ester by looking at the structural formula. There are only three types of compounds which have an oxygen located between two carbons. These are ethers, esters and acid anhydrides. Can you identify the differences? Second, we will perform a hydrolysis reaction. Hydrolysis occurs when water is used to break a bond. Hydrolysis can occur in either basic or acidic conditions. We will learn later the actual mechanisms, but in both cases we produce one chemical which is an alcohol and the other chemical will be a carboxylic acid when esters are hydrolyzed. Third, we will produce an ester. It should be noted that ester formation cannot occur in the presence of base, only acid.
During the first day of this lab, we will produce salicylic acid. This acid will then be used to make aspirin. The synthesis of aspirin is a multi-billion dollar a year chemical. While salicylic acid as some therapuetic value, it is not as effective as aspirin in reducing inflammation and other common medical conditions for which aspirin can be used. Aspirin is part of a large group of chemicals classified as Non-Steriodal Anti-Inflammatory Drugs (NSAIDS).
This experiment is composed of two parts. The first involves the hydrolysis of methyl salicylate in order to produce salicylic acid (Day 1). The salicylic acid produced in this part of the experiment will be used to prepare acetylsalicylic acid (aspirin) (Day 2). The aspirin must be very pure, so you will do a second purification of the aspirin on the third day.
Many esters have familiar odors. Methyl salicylate, an ester derived from the combination of salicylic acid and methanol, is also known as the oil of wintergreen. Methyl salicylate was first isolated in pure form in 1843 by extraction from wintergreen plant (Gaultheria). It was soon found that this compound had analgesic and antipyretic character almost identical to that of salicylic acid when taken internally. This medicinal effect probably results from the ease with which methyl salicylate is hydrolyzed to salicylic acid under the alkaline conditions found in the intestinal tract. Methyl salicylate can be taken internally or absorbed through the skin, hence its use in some liniment preparations. When applied to the skin, it produces a mild burning or soothing sensation, which is probably due to the action of its phenolic hydroxyl group. Methyl salicylate also has a pleasant odor, and it is used as an extract for flavoring purposes.
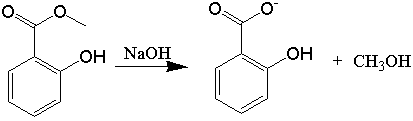
Esters can be hydrolyzed into their carboxylic acid and alcohol components under either acidic or basic conditions in the presence of heat. In this experiment, methyl salicylate, an ester also known as oil of wintergreen because of its natural source and odor, is treated with aqueous base and heated. Since, in our experiment, hydrolysis occurs in the presence of base (instead of acid), the carboxylic acid and phenolic -OH groups on salicylic acid are ionized and this compound exists as the sodium salt of salicylic acid, sodium salicylate. The reaction mixture is subsequently acidifed using sulfuric acid, which converts this anion into the fully protonated acid, salicylic acid. The alcohol is methanol. The salicylic acid, which is mostly insoluble, is a solid and can be isolated and purified by crystallization.
The chemical equation that describes this experiment is:

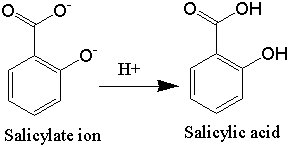
As mentioned above, the phenolic hydroxyl group, which is also acidic, would be ionized and exist as the sodium salt during the basic hydrolysis, just like the carboxylic acid group, but it is not shown ionized in this figure because we are concerned with the ester hydrolysis. As the following figure shows, the phenolic -OH, as well as the carboxylic acid group, will be protonated during the acidification step following the addition of the sulfuric acid. The following figure shows what happens during acidification:
The hydrolysis of methyl salicylate will be performed using the following setup.
Heat the reaction mixture to boiling using a heating mantel. Allow the mixture to reflux (with cooling) for about 20 minutes. The liquid mixture should be continually boiling for the entire reflux time.
After a 20 min reflux, transfer the reaction mixture to a 250-mL beaker.
Store the salicylic acid crystals in an evaporating dish or beaker in the drying oven until the next lab period. Since your collected crystals from an acid solution, you cannot store your filter paper with your chemical.
STOP AND STORE THIS PART OF THE EXPERIMENT UNTIL THE NEXT LAB PERIOD!
Aspirin is a trade name for acetylsalicylic acid, a common analgesic. Acetylsalicylic acid is an acetic acid ester derivative of salicylic acid. The earliest known uses of the drug can be traced back to the Greek physician Hippocrates in the fifth century B.C. He used powder extracted from the bark of willows to treat pain and reduce fever. Salicin, the parent of the salicylate drug family, was successfully isolated in 1829 from willow bark. Sodium salicylate, a predecessor to aspirin, was developed along with salicylic acid in 1875 as a pain reliever. Sodium salicylate was not often popular though, as it has a habit of irritating the stomach. However, in 1897, a man named Felix Hoffman changed the face of medicine forever. Hoffman was a German chemist working for Bayer. He had been using the common pain reliever of the time, sodium salicylate, to treat his father's arthritis. The sodium salicylate caused his father the same stomach trouble it caused other people, so Felix decided to try and concoct a less acidic formula. His work led to the synthesis of acetylsalicylic acid, or ASA. This soon became the pain killer of choice for physicians around the globe. Scientists never really understood the inner workings of the drug however. It wasn't until the 1970's, when British pharmacologist John Vane, Ph.D. began work on aspirin that people began to understand how aspirin really works. Vane and his colleagues found that aspirin inhibited the release of a hormone like substance called prostaglandin. This chemical regulates certain body functions, such as blood vessel elasticity and changing the functions of blood platelets. Thus can aspirin affect blood clotting and ease inflammation.
The reaction for synthesis of acetylsalicylic acid is shown in the following figure. Salicylic acid, prepared from the hydrolysis of methylsalicylate is reacted with acetic anhydride producing the ester product, acetylsalicylic acid.
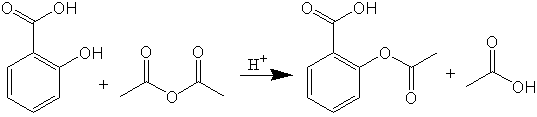
In a previous experiment, we have used the Fischer esterification reaction to produce isopentyl acetate from an acid (acetic acid) and an alcohol (isopentyl alcohol). The current experiment uses the carboxylic acid derivative, acetic anhydride, for ester formation. The advantage of using acetic anhydride is that you do not produce water which can be used for hydrolysis of the newly formed ester. Concentrated sulfuric acid will be used to keep everything in the protonated state. Acetic anhydride is the preferred acid derivative to synthesize aspirin commercially because the acetic acid produced in this reaction (a reaction by-product) can be used again, by converting it back into acetic anhydride.
If you recovered less than 3.5 g of salicylic acid, you will need to adjust the quantities of reagents used in this protocol. However, if you recovered at least 3.5 g salicylic acid, use the quantities of reagents listed below (remember, if you recovered more than 3.5 g of salicylic acid, use only 3.5 g salicylic acid regardless of how much you recovered). If you recovered less than 3.5 g salicylic acid, just proportion the amounts of the other reagents for this reaction.
Thoroughly cool the flask to complete the initial crystallization of aspirin. Remember that when a solution becomes cloudy, that is a solid forming and this would be the crystals of aspirin you desire. If crystals are slow in forming, you may need to scratch the inside of the Erlenmeyer flask with a glass rod, which will speed up crystal formation by seeding, or initializing the formation of crystals. Collect your aspirin via vacuum filtration, and let air continue to be drawn over the solid for about 10 minutes to evaporate most of the water surrounding the solid.
The aspirin product will be analyzed using HPLC (High Pressure Liquid Chromatography). The HPLC resolves the aspirin from the salicylic acid. For your analysis, weight out precisely 0.0100 grams of sample and place in a clean test tube. To this test tube, add five mL methanol, and swirl to dissolve aspirin. Then, add 5 mL Milli-Q water (ultra purified) to facilitate dissolving. This dissolved material will be given to an IA who will run your sample on the HPLC and give you a copy of the chromatogram of your crude aspirin.
Take about 2 grams of your slightly moist aspirin isolated above and add to a small beaker. Add either 5 mL of methanol or 5 mL of acetone and gently swirl to dissolve the solid. You may need to warm slightly, but do not boil the mixture. All of the aspirin should dissolve in the warm solvent. Remove the beaker containing the aspirin-solvent mixture and let it cool to room temperature on the bench. When it is about room temperature (the beaker will feel slightly cool to the touch), place the beaker in an ice bucket to continue to produce crystals. After about 15 minutes on ice, collect by vacuum filtration your crystals (do not use water to wet the filter paper, but use the same solvent to wet the filter paper prior to collecting crystals).
Let the vacuum continue to be drawn through the Buchner funnel for about 15 minutes to thoroughly evaporate the solvent.
At this point, your filter paper and your crystals should be dry. Weigh your sample to determine your yield of re-crystalized aspirin. For HPLC analysis of your re-crystallized aspirin, weigh out about 0.0100 grams of your re-crystalized aspirin and add 5 mL of the anhydrous methanol, dissolve and then add 5 mL Mili-Q water to dissolve your solid. Slight warming should not be necessary.
Do a yield, percent yield, and melt temp of your re-crystallized aspirin. Be sure to have your aspirin samples analyzed via HPLC.
Caution: Aspirin decomposes in boiling water (therefore, do not boil the aspirin) or when dissolved in solutions of alkali hydroxides and carbonates. Inorganic salts of acetylsalicylic acid are soluble in water (especially the Calcium salt, but are decomposed quickly.
An alternative re-crystallization procedure could include one of the following options:
What is the yield of dry aspirin? What is the percent yield? What is the melt point? (The crystals may have a wide melting range, from 125-138oC, because of potential of decomposition. The use of a preheated melting point device (heated to about 110oC) will help to minimize this decomposition. Why do you think this could help prevent decomposition?)
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Methyl salicylate | 152.15 | 6.4 mL (7.5 g) (use pipettor) | 49.3 | -8.6 | 223.3 | 1.184 | msds | |
| Salicylic acid | 138.12 | 3.5 g | 25.3 | 159 | 211 | 1.44 | msds | |
| Acetyl salicylic acid (aspirin) | 180.16 | 135 | 140 | 1.35 | msds | |||
| Acetic anhydride | 102.09 | 3.5 ml (use pipettor) | -73.1 | 139.9 | 1.08 | 1.389 | msds | |
| NaOH (5 M) | 40.0 | 25 mL | 125 | msds | ||||
| H2SO4 (6 M) for Day 1 | 98.0 | 8-10 mL | msds | |||||
| H2SO4 (18 M) for Day 2 | 98.0 | a few drops | msds | |||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
Go To Experiment: ChemDraw 1
2
3 4
5 6
7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright Donald L. Robertson (Date last modified: 11/14/2012)