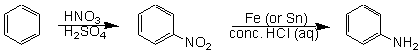
One of the most generally useful methods of introducing an amimo group into a molecule is to introduce a nitro group first, and then reduce the nitro group to the amine. For instance, the best way to make aniline (and many aniline derivatives) is to make nitrobenzene first, and then reduce nitrobenzene using iron or tin, and hydrochloric acid:
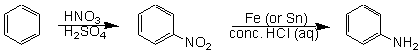
It is also possible to reduce any nitro group using hydrogen and a standard hydrogenation catalyst, such as platinum or palladium:
![]()
In this experiment you will reduce an already existing nitro group to the amine, using tin and hydrochloric acid:
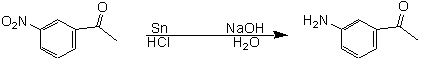
Some of the chemicals used in this experiment are potentially dangerous. Please pay attention to the following Safety Precautions.
Safety precautions:
Combine 6.05 mmol (that's "millimoles", not "moles") m-nitroacetophenone and 16.9 mmol granulated tin in a round bottom flask. Add a stir bar to the flask, and set up the flask for a reflux. To prepare dilute HCl (3M HCl), add 5 mL concentrated hydrochloric acid (12.0 M) with 15 mL water. Add your freshly prepared 3M HCl to the round bottom flask, and reassemble the reflux setup (if 3 M HCl is already available on the cart, you can use 20 mL of this solution, instead of making your own). Reflux (with stirring) the mixture long enough to dissolve most of the tin (about 30 minutes).
While the reflux is proceeding, dissolve 5 g of sodium hydroxide in 12 mL of water in a beaker or Erlenmeyer flask (it will get hot while dissolving). Set the container aside to cool (after all the pellets have dissolved). When it is cooled to room temperature, put the container in an ice bath (use a larger beaker for the ice bath that is big enough for a round bottom flask to fit in) to lower the temperature to 0oC.
Disassemble the reflux assembly and allow the flask to cool to near room temperature (set it on a cork ring to cool). After the flask is cooled to room temperature, place it into your previously prepared ice bath (remove the cold NaOH from the ice bath) for a few minutes to cool to about 0oC. Place the entire ice bath, with your sample, onto the stirring hot plate (no heat, of course). Clamp the round bottom flask so that it sits upright in the ice bath on the stirring hot plate. Add all of the cold NaOH solution to the reaction mixture at once using a funnel. Stir the mixture while it sits in the ice bath, for about 10 minutes.
Collect the solid product by suction filtration, using only a small amount of ice cold water for washing. There is no need to purify your product further today. Let your product dry until the next lab period.
In preparation for the next lab period, if you need additional materials to effectively separation the tin (or is insoluble hydroxide) from your desired organic compound, you must request these additional chemicals, solvents, or other items from the stockroom during the first day (yellow check-out sheet). Requests made during the second day cannot be honored, although if the chemical is already out, because another student requested it, you can use it.
It is possible, or, rather, highly likely, that your solid material will contain some tin in the form of tin hydroxide. This may be a metallic grey color (if all the Sn did not dissolve and become cation). You must devise a protocol to get rid of the tin hydroxide from the organic product (see footnote #1).
Remember, you must design your separation protocol to isolate the organic product. Use procedures you have used before in previous labs.
For informational sake, you might think about the following. You could make your product soluble by adding acid (protonating your amine), but acid will also dissolve your tin(IV) hydroxide. If you make the mixture basic, your product will become less soluble, but the tin(IV) hydroxide will also be less soluble. So, that is the dilemma. How can you solve it?
You can use any of the following (not necessarily the only reagents or solutions you could use) chemicals:
Determine the yield, melting point, and take the IR spectrum (do an IR only if requested by your instructor). Show your solid material to the instructor (are you following instructions?). If your sample is not the correct color, you will be deducted points.
Percent Yield Calculation: To determine the percent yield, you must first determine a theoretical yield. To determine theoretical yield, remember that one mole of m-nitroacetophenone will produce one mole of m-aminoacetophenone. Therefore, the theorectical yield would be based on a simple set of ratios: grams of starting material divided by molar mass of starting material is equal to theorectical yield divided by the molar mass of product. This is a simple equation.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| m-nitroacetophenone | 165.1482 | 1.00 g | 6.05 | 76 - 78 | 202 | --- | --- | msds |
| granulated tin | 118.69 | 2.00 g | 16.9 | 231.9 | 2270 | 7.3 | --- | msds |
| NaOH | 40.0 | 5 g | 125 | --- | --- | msds | ||
| m-aminoacetophenone | 135.1652 | 97 | 290 | msds | ||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
Footnote #1:
Go To Experiment: ChemDraw 1
2
3 4
5 6
7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright Donald L. Robertson (Date last modified: 11/14/2012)