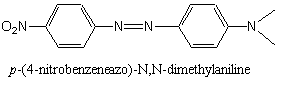Experiment 8
Diazonium Coupling Reaction of p-Nitrobenzenediazonium sulfate and
N,N -Dimethylaniline: Synthesis of p-(4-nitrobenzeneazo)-N,N-dimethylaniline
Objectives
Amino groups on aromatic rings especially are convenient because they can be
used to attach many other types of groups to the ring. For example, we can
attach halogens, we can remove the amino group completely, and we can attach the
hydroxy group to produce a phenol. Often starting with a nitro group, we can
reduce it to an amino group, and then carry out other modifications.
Background
Diazonium salts are very useful synthetic intermediates; they can be converted
to a large number of functional groups, and, in many cases, are used as the key
reaction step in the only practical synthetic route to yield substituted benzene
compounds. Another set of important synthetic uses for diazonium salts is the
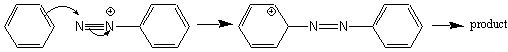
diazonium coupling reaction. The terminal nitrogen of the diazonium cation is
used as an electrophile in an EAS reaction:
Most of the products of this type of diazonium coupling reaction are brightly
colored, and many of the dyes used in textiles in the past century are made in
this way. These dyes belong to the class of chemicals called "azo-dyes." As
a dye, these chemicals tend to stick to objects, including clothing and your
skin. To avoid "dying" items you don't wanted dyed, wear gloves when handling
any of these colored compounds.
Safety: This experiment uses many dangerous substances -- use extreme
care!
-
Sulfuric acid is highly corrosive, toxic, and a
strong oxidizing agent -- wear gloves while handling it.
-
p-nitroaniline is highly toxic and an irritant -- wear
gloves while handling it.
-
Sodium nitrite is toxic and an oxidizing agent -- wear
gloves.
-
N, N-dimethylaniline is highly toxic and a cancer
suspect agent -- wear gloves while handling it, and measure it and use
it in the hood.
-
Sodium hydroxide solution is corrosive and toxic
-- wear gloves while handling it.
In all cases, report any spills immediately to your instructor. After you
have informed the instructor, you should immediately clean up the spill
completely. Your instructor can assist you.
Procedure
Day 1 (Parts A and B must be done the first day)
Part A: Preparation of p-nitrobenzenediazonium sulfate
The preparation of p-nitrobenzenediazonium sulfate is accomplished as
follows.
-
Make a solution consisting of 2 mL concentrated H2SO4
diluted into 10 mL H2O (always add acid to water).
-
Add 1.38 g of p-nitroaniline to the freshly prepared sulfuric acid
solution.
-
Heat the mixture gently to dissolve the p-nitroaniline that you just
added.
-
Cool the solution to about room temperature, but it is best if a lot of
solid is not allowed to reform. If necessary, your mixture can be
cooled in an ice bath, but the mixture should be maintained at about 10-15°C
if possible; be careful that you do not cool it too much, trying to keep the
solid insolution, without solid residue forming.
The formation of the diazonium ion (a salt when it combines with the sulfate
ion) is formed using the following protocol:
- Make a solution of NaNO2 by adding 0.69 g NaNO2 to
about 2 mL of water in a test tube
- Add the aqueous NaNO2 drop-wise to the previously prepared
p-nitroaniline-H2SO4
mixture
- After you add a few drops of NaNO2 solution, mix the
reactants using swirling, a stirring rod or a stirring bar.
- This solution, when combined with the p-nitroaniline mixture
forms the nitrosyl cation required for the formation of the diazonium
salt.
Keep the temperature of the reaction mixture below 10-15°C at all times (but not
ice cold). After the addition of the NaNO2 solution is
complete, you can store your mixture on ice until you are ready to use it in
Part B.
Part B: Reaction of p-nitrobenzenediazonium sulfate with N, N-dimethylaniline
The p-nitrobenzenediazonium sulfate prepared in Part A will now be
used to prepare your desired product.
-
Make a solution of 1.21 g N,N-dimethylaniline (dispensed in the hood;
convert mass to volume using density) in 1.5 mL of aqueous 1 M HCl
(based on its density, what volume of N,N-dimethylaniline do you
need?).
- Use a pipetting device to make this addition, don't use a graduated
cylinder, because your chemical will adhere to the glass.
- Cool this solution in the ice bath (never us a graduated cylinder as a
reaction container)
- Transfer this solution into a beaker for later reaction
-
Add small amounts of the cold reaction mixture from Part A
(p-nitrobenzenediazonium sulfate) to a small beaker containing the
freshly prepared N,N-dimethylaniline and HCl solution.
-
Mix well after each addition (note any color changes)
-
After the mixing of chemicals is complete, add about 10 mL of cold aqueous 1
M
NaOH.
A solid should form which should be intensely colored.
- Collect the reddish-brown product by Büchner funnel filtration as usual.
(Use water to rinse your beaker.)
- Remove the solid product you just collected and transfer to a clean
beaker (scrape the solid off of the filter paper).
- Add water to suspend the dye and mix thoroughly.
- Collect this washed, suspended solid using second Büchner funnel
filtration (this wash ensures removal of non-organic material such as salts
or base)
Store the solid dye in the drying oven until the next lab period.
Day 2
The structure for the anticipated chemical product is shown below.
Remove your dry dye from the drying oven. Determine yield, percent yield and
melting point.
Put all of the product in a labeled vial and give it to the instructor if
requested (or you can collect the lab supply of this chemical and use it to dye
clothes at home).
J
Chemicals, Reagents, and Supplies
| Compound |
MW |
Amount |
mmol |
mp |
bp |
Density |
ηD |
msds |
| p-nitroaniline |
138.1256 |
1.38 g |
10.0 |
146 |
332 |
--- |
--- |
msds |
| p-nitrobenzenediazonium sulfate |
396.2 |
--- |
--- |
--- |
--- |
--- |
--- |
|
| N,N-dimethylaniline |
121.1816 |
1.21 g (1.3 mL)
(use pipettor) |
10.0 |
2.45 |
194 |
0.956 |
1.5581 |
msds |
| p-(4-nitrobenzeneazo)-N,N-dimethylaniline1 |
270.266 |
--- |
--- |
2291 |
--- |
--- |
--- |
|
| Compound |
g/mol |
grams or mL |
10-3 mol |
oC |
oC |
g/mL |
ηD |
msds |
1The melting point of ultra-pure dye is 229oC. The dye with impurities actually starts to decompose at about 159oC with a melt point range typically of from 159-190oC. Report what you observe.
Questions:
-
Show the reaction necessary to convert the nitrite ion into nitrous acid.
Show how nitrous acid can be used to produce the nitrosyl cation.
-
Draw a second resonance structure of the p-nitrobenzenediazonium ion,
showing the terminal nitrogen as the electrophilic nitrogen (possessing a
formal charge of +1).
-
Assuming that you had a quantitative conversion of p-nitroaniline
into p-nitrobenzenediazonium sulfate, how much of this product should
you have produced?
-
What is the most logical explanation as to why you do not produce any
of the ortho- or meta-substituted product (there may be
different explanations for each substitution possibility)? Why is the only
product shown as being the para-substituted product?
-
What is the percent yield of
p-(4-nitrobenzeneazo)-N,N-dimethylaniline? What is the
melting point of your product? Does it have a precise melting point?
Copyright Donald L. Robertson (Date last modified:
11/14/2012)