Experiment 4
Williamson Ether Synthesis
Objectives
Ethers can be produced from two alcohols. However, unless you want to
have symmetrical ethers (e.g., diethyl ether derived from ethanol), ether
synthesis from different alcohols in the same reaction mixture will produce a
variety of products. To produce an unsymmetrical ether (e.g., t-butyl
methyl ether), you can do this if one component as an alkyl halide and the other
component is the alkoxide (or phenoxide) ion. The alkoxide ion can be any
alkoxide but the alkyl halide is usually going to be a primary or methyl halide.
The reason for this is that a primary halide would have less chance of
undergoing elimination, hence you can end with the product you want. In
this experiment we will use p-cresol and chloroacetic acid. The alcohol can
easily be converted into the phenoxide ion. This procedure is typical for
reactions used to produce asymmetrical ethers. The Williamson Ether
synthesis is one of several organic chemistry reactions referred to as Named
Reactions, which employ the name of the scientist who developed it.
Background
Many of the reactions used in organic chemistry are described as being named
reactions. The Fischer Esterification reaction was a named reaction, referring
to Emil Fischer who discovered and popularized it as a method to produce esters.
Likewise, the Grignard Reaction was similarly named after its discoverer.
In today's experiment, the Williamson ether synthesis is another named reaction,
developed by Dr. Alexander W. Williamson who was a professor at University
College in London in the latter part of the 1800's. This reaction has been
around for a long time and has been used successfully to synthesize many
different ethers. For this reaction to occur at a high yield, the alcohol
portion can be either 1o, 2o, or 3o, which can
then be converted into an alkoxide nucleophile using basic conditions (e.g.,
NaOH can be used as the base). The alkoxide ion then reacts via a SN2
reaction mechanism with a primary alkyl halide (in the current experiment, we
will use chloroacetic which also cannot undergo elimination). For example, if
the alkyl halide was either 2o or 3o, an E2 elimination
reaction would likely take place instead of substitution.
Procedure
The simplest way to synthesize an ether is to have an alkoxide react with a
primary haloalkane or a sulfonate ester under typical SN2 conditions.
The ether prepared in this experiment is a methylphenoxyacetic acid, which is a
phenolic (benzene ring attached to something is a phenyl group) ether that is
prepared from p-methylphenol (cresol) and chloroacetic acid.
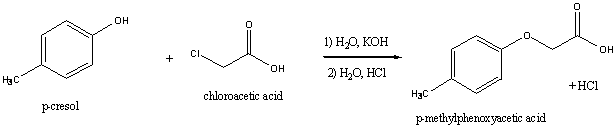
The methylphenoxyacetic acid family is of interest for several reasons,
including the following:
- The products are easily prepared crystalline solids, which serve as
solid derivatives whose melting points can be used to identify the liquid
phenol starting materials (o- or m- or p-cresol).
- Several well-known herbicides are members of this class of compounds,
especially 2,4-dichlorophenoxyacetic acid (2,4-D) and
2,4,5-trichlorophenoxyacetic acid (2,4,5-T). These herbicides mimic the
effect of the natural plant growth regulators, known as auxins, causing the
plant to grow too rapidly. These herbicides are fairly selective toward
broadleaf weeds, such as dandelions, velvetleaf and plantain. Agent Orange,
a mixture of the butyl esters of 2,4-D and 2,4,5-T, was used by the U.S.
troops as a defoliant during the Vietnam War.
We will carry out the reaction shown above to illustrate the Williamson ether
synthesis and to identify (if different isomeric cresols were used) by the
melting point of the product.
To perform this synthesis, do the following:
- Dissolve 4 g of KOH pellets (cannot use NaOH) in 8 mL of water in a
250-mL round bottom flask (use the flask that contains two ground-glass
openings (one for the condenser unit and the other for the Separatory
funnel).
- Add 2 grams of your cresol to the round bottom flask in the hood.
- Swirl the mixture until a homogeneous solution results.
- Add 3 boiling stones.
- Fit the flask with a reflux condenser and heat to a gentle boil.
- After the mixture has started to boil, add 6 mL of a 50% aqueous
solution (g/mL) of chloroacetic acid drop-wise using a Separatory funnel
placed in the side-arm of your round-bottom flask.
- After addition of the chloroacetic acid solution, continue continue
refluxing for another 10 minutes.
- After reflux, transfer the solution to a small beaker while it is still
hot (if you let the mixture cool, solid forms inside the flask and it is
more difficult to remove). You can overcome this problem by adding about 10
mL of DI water to the reaction mixture, and then pour this slightly diluted
mixture into a 100-mL beaker..
- After you have poured the (diluted) reaction into the 100-mL beacker,
let it cool to room temperature in the beaker.
- Acidify the solution with drop-wise addition of concentrated 12 M
HCl. (Monitor the pH with pH/litmus paper to be certain pH is acidic).
- It might be beneficial to ensure you do not isolate the potassium salt
to heat the acidified mixture with solids until it all dissolves at the
higher temperature. Then, simply cool that mixture in an ice bath to
attain maximal yield of solid product. Be sure the precipitate has formed
completely, perhaps storing on ice for about 15 min before filtering it.
- Filter and collect the precipitated product by using a Buchner funnel
vacuum filtration setup.
- To the collected solid product from the previous step, you will perform
a re-crystallization step by adding the entire crude product to boiling
water. Do not use more than 50 mL of water to ensure you will be able to
isolate crystalline product.
- Collect your re-crystallized product using vacuum filtration.
- Allow the solid to dry until the next lab period in the designated
drying oven.
(Be certain to label your containing appropriately using all of the
information listed on the doors of the drying ovens.)
- Weigh the solid to determine overall yield. From this yield you should
be able to determine a percent yield based on what you recovered solid was
and the theoretical yield for this experiment. Also, determine the melting
point after you have determined your yield for recovery of your product.
Hand in a properly labeled sample of your product. Include your name, melting
point of product, name of product.
Chemicals, Reagents, and Supplies
| Compound |
MW |
Amount |
mmol |
mp |
bp |
Density |
ηD |
msds |
| p-cresol (p-hydroxytoluene) |
108.13 |
2.0 mL (2.070 g) |
19.2 |
34.8 |
201.9 |
1.035 |
|
msds |
| chloroacetic acid (50%; mass/vol) |
116.48 |
3.0 g (6 mL 50% - m/v) |
25.8 |
170 |
--- |
|
|
msds |
| KOH (pellets) |
56.11 |
4.0 g |
71.3 |
380 |
--- |
|
|
msds |
| HCl (12 M; 12 mmol/mL) |
36.45 |
6-8 mL |
|
|
|
|
|
msds |
| p-methylphenoxyacetic acid |
166.17 |
--- |
|
140-142 |
|
1.327 |
|
msds |
| o-methylphenoxyacetic acid |
166.17 |
--- |
|
152-154 |
|
1.327 |
|
msds |
| 3-methylphenoxyacetic acid |
166.17 |
--- |
|
102-103 |
|
1.327 |
|
msds |
| 2-naphthol |
144.17 |
2.77 g |
19.2 |
121-123 |
285-286 |
1.22 |
|
msds |
| methanol |
32.04 |
10.0 mL |
|
-98 |
64.6 |
0.791 |
1.3286 |
msds |
| diethyl ether |
74.1224 |
~20 mL |
|
-116.3 |
34.6 |
0.7134 |
1.3526 |
msds |
| Compound |
g/mol |
grams or mL |
10-3 mol |
oC |
oC |
g/mL |
ηD |
msds |
Product Synonyms:
- Ether: Diethyl Oxide; Ether; Diethyl ether; Ethyl ether;
1,1'-Oxybisethane; ethyl oxide; pronarcol; Ethoxyethane; Ethyl Ether;
DIETHYL ETHER (ETHYL ETHER);
QUESTIONS
- Sodium hydroxide reacts with β-naphthol to form the sodium salt. Can
sodium ethoxide, the sodium salt of ethanol be formed the same way? If not,
what is an effective way to produce sodium ethoxide which can be used in
organic reactions?
- Explain why phenols, including the β-naphthol, which is used in the
current experiment, would be acidic and why it easily reacts with base.
- Why does the ether precipitate when the alcoholic reaction mixture is
poured into water?
- What else could have been used in place of ethyliodide to produce the
same ether?
- Outline the SN2 mechanism for this reaction.
- Why is sodium hydroxide used in the smallest molar amount?
- Explain the process of the "seeding" procedure.
Copyright Donald L. Robertson (Date last modified:
11/14/2012)

