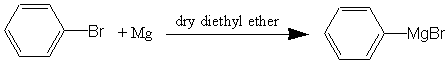
One of the most important things an organic chemist can do is to make a compound which has a different number of carbons in it than what he/she started with. Most of the reactions in the past have involved simply substitutions or combining different compounds. The reaction today is designed to make new compounds having addition carbons in their structure. The Grignard Synthesis is useful for adding carbons to molecules that have a carbonyl carbon. Depending on the compound you start with, you either produce alcohols or acid-derivatives. In this experiment you will start with either an aldehyde or ketone and produce an alcohol. Alcohols as you will observe later are one of the most useful organic compounds because they are easily converted into many different types of compounds. Since the Grignard Synthesis uses a carbanion as an intermediate (carbanions are extremely strong bases), any protic acids (e.g., water and alcohol) must not be present because the strong carbanion bases would abstract a proton (H+) from these acids and become non-functional as a Grignard Reagent. Therefore, your reaction containers and reagents must be free of water or other protic solvents or reagents (there would be enough acid in a finger-print to destroy your reagent).
In this experiment, you will prepare the Grignard reagent (or use previously prepared chemical), phenylmagnesium bromide.
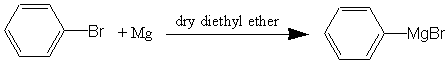
Phenylmagnesium bromide will be used to produce either benzoic acid (reaction with CO2; dry ice) or triphenylmethanol when reacted with benzophenone (or ethyl benzoate). The organic portion of the Grignard reagent functions as a carbanion nucleophile. After reaction, the desired product (an alcohol, as shown here) is formed after acidic hydrolysis.
![]()
You must keep your reaction apparatus and reagents completely dry because water functions as an acid, and would cleave the Grignard reagent producing benzene. All glassware must be thoroughly clean and dry. After washing any glassware, you should remove any visible appearance of water with a heat gun, and then bake your glassware for at least 30 minutes at 110oC. While you are drying your glassware, you should assemble a drying tube containing CaCl2 and place it in the oven too. Be certain not to put any plastic items into the oven because they could melt or become distorted.
Please note that the Grignard reagent that you prepare, is used for two separate reactions. The first is for the synthesis of benzoic acid (Part A) and the second if for the preparation of triphenylmethanol (Part B). After the Grignard reagent is prepared, both Part A (Day 1) and Part B (Day1) experiments must be performed. In other words, all of the Grignard reagent will be reacted the day it is made, half of which is used in Part A in a beaker containing the dry ice, and the other half used in Part B, in the same glassware as used to prepare the Grignard reagent.
At the discretion of your Instructor, phenylmagnesium bromide, will either be prepared using the procedure outlined below, or your Grignard reagent will already be prepared. If commercially available Grignard is to be used, you should proceed directly to Parts A & B for Day 1 of the experiment. The reactions outlined in Parts A & B are the same regardless of your source of Grignard reagent.
The procedure outlined here is how a Grignard reagent is prepared. For the lab today, instead of making your own Grignard reagent, you will be using commercially available chemical. The procedure for synthesis is included here in order to show you the complete way of making the chemical. Since the reagent is available, proceed directly to doing Parts A & B.
The reaction apparatus is setup as follows (all items must be clean and dry):
(The other glassware, such as beakers and graduated cylinders, which will be used for dispensing and measuring the anhydrous ether, should also have been baked.)
The reaction is set up and performed as follows (Prepare an ice bath to cool the solution if the reaction becomes too vigorous):
Cloudiness and bubbles at the surface of the metal are indicators that the reaction has begun. This may take up to 5-10 minutes. If no reaction is apparent at the end of this 10-minute period, add a few small crystals of iodine. If the reaction still doesn't start, consult the instructor, and you will likely need to start over.
Once the reaction has started, turn on the cooling water in the condenser and start the stirrer. Add the remainder of the bromobenzene/diethyl ether solution slowly, and drop wise, to maintain a steady reflux. Too rapid addition might result in the formation of diphenyl (draw the structure and predict the reaction mechanism). This addition step should take about 45 min. After the bromobenzene solution has been added, rinse the addition funnel with an additional 3 mL of anhydrous diethyl ether and add this rinse to the round-bottom flask.
Fit the flask with a heating mantle and reflux gently for an additional 15 min. The final Grignard mixture should be cloudy and most of the Mg metal should be gone. Cool the mixture to room temperature and then add enough anhydrous diethyl ether to give a final of volume of about 40 mL in the reaction flask. The Grignard reagent must be used immediately after it has cooled.
You will use the Grignard reagent for the two reactions described in Parts A and B.Use about half (~20 mL) of the freshly prepared phenylmagnesium bromide (Grignard reagent) for each of these reactions (you do not need to measure precisely in a graduated cylinder).
The Grignard reagent must be used the day it is prepared, and the Day 1 section for each experiment must be performed the same day you made the Grignard reagent.
Caution: Diethyl ether is volatile and an anesthetic (it can make you dizzy and lightheaded). At no time is ether allowed to be in the open lab unless it is in a covered, or closed container (e.g., stoppered separatory funnel or other container). Always dispense ether in the hood, and replace the lid immediately after use. Use of ether in any open container must be conducted in the hood (e.g., ether in a beaker, or during filtrations). Ether can be used in a separatory funnel outside the hood. No exceptions will be allowed!
Reaction of phenylmagnesium bromide with benzophenone is shown below. This reaction, like the reaction to produce the benzoic acid must be completed the first day, immediately after preparing the Grignard reagent.
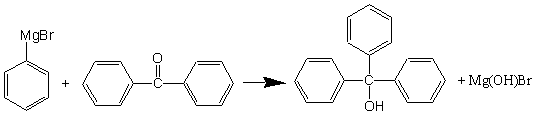
Preparation of triphenylmethanol.
Cool the reaction only if it is required to control the mildly exothermic reaction. Evidence of reaction will be the formation of a bright reddish-pink solution which will eventually precipitate as a white solid.
The reaction is completed using a gentle reflux for about 15-30 minutes. You should observe solid material in the round-bottom flask which will continue to accumulate during reflux.
After your reflux has finished, transfer the contents of the entire 100-mL reaction flask to a 125-mL Erlenmeyer flask. Add about 10 mL of DI water to your flask (don't worry because your reaction is already complete) and let the entire contents of the Erlenmeyer flask sit in the hood until the next lab period. Do not seal the flask, but allow it to remain open to the atmosphere to facilitate THF and diethyl ether evaporation until the next lab period. Place your labeled flask into one of the storage containers which will ultimately be placed in the hood in the the stockroom.
STOP AND STORE THIS PART OF THE EXPERIMENT UNTIL THE NEXT LAB PERIOD!
The formation of benzoic acid is shown below.
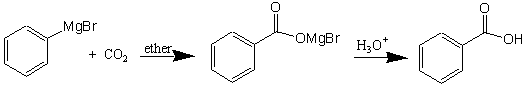
Add about 5-10 grams (do not weigh, since excess sublimes) of crushed dry ice (use large mortar and pestal) to a 250-mL beaker. Add about 15 mL of the 1 M Grignard reagent (phenylmagnesium bromide) in tetrahydrofuran (THF) to your beaker containing the dry ice. There should be obvious signs of mixing which includes gas bubbling from the dry ice when the Grignard reagent is added. This gas is carbon dioxide, but your Grignard reagent will be reacting with the dry ice in the beaker. After you have added the phenylmagnesium bromide, let the beaker sit at room temperature until all the dry ice has sublimed and the frozen solid has melted. The Grignard reaction product should appear as a viscous glassy mass with a brownish color.
You will need to hydrolyze the Grignard adduct by slowly adding about 10 mL of 3 M HCl with stirring (you can use a glass stirring rod or simply swirl the contents of the beaker by hand). After the addition of your HCl, monitor the pH to make certain that the pH is about 1 using pHydroin paper. If the pH is higher than desired, continue adding a little more 3 M HCl to lower the pH. At this low pH, all of the benzoic acid should be protonated.
Continue to mix the contents of your beaker using a glass stirring rod or by swirling its contents. Since THF is mostly soluble in water, you can enhance its evaporation by letting the contents of the beaker sit in the hood at room temperature until the next lab period. Solid benzoic acid will be apparent even while some THF is present. Letting your sample sit until the next lab period will leave the benzoic acid suspended in the aqueous layer. Even if some or all of the water evaporates, this will not affect your ultimate yield or product.
STOP AND STORE THIS PART OF THE EXPERIMENT UNTIL THE NEXT LAB PERIOD!
Your triphenylmethanol product should be be free of THF and diethyl ether after sitting until this second lab period.
Add about 25 mL of 1.0 M sulfuric acid to your 125-mL Erlenmeyer flask containing the triphenylmethanol. Transfer the contents of your Erlenmeyer flask to your Separatory funnel remembering to always use a funnel when pouring through ground glass openings. Add about 20 mL of diethyl ether (it does not need to be anhydrous diethyl ether from this point forward, but it is okay) and up to 5-10 mL of 1.0 M sulfuric acid to rinse the Erlenmeyer flask of remaining crystals. Swirl the flask well to promote hydrolysis (the acid converts the alkoxide ion into an alcohol) of the addition product.
Pour the wash liquid from your flask into the Separatory funnel. You may use up to 10 mL of diethyl ether to remove any remaining crystals. Shake the Separatory funnel vigorously for up to 5 minutes and let the two phases separate (if all solids have not dissolved add a little more diethyl ether). Remove and discard the lower aqueous layer, which does not contain your triphenylmethanol, since it is more dense than the ether phase. If in doubt, simple add a little distilled water, and see which phase gets larger, which would then be the aqueous phase. After removal of the aqueous phase, add about 10 mL of the 1.0 M sulfuric acid to wash the diethyl ether phase. As a last extraction/wash step, add about 10 mL of saturated NaCl to enhance the removal of any water dissolved in the ether (the salt solution also allows the aqueous and ether layers to separate more effectively).
Note: It is important to note that quantities of the wash liquids is not critical, and it is usually sufficient to use about one-third to one-half of the volume of the ether for each wash with the aqueous solutions.
To effect drying of the ether solution (removal of any dissolved water in the ether), decant the ether layer from the Separatory funnel into an 125-mL Erlenmeyer flask. Add about 2 g of anhydrous Na2SO4 and swirl the flask from time to time. If it appears that the sodium sulfate crystals are clumping together, add a little more anhydrous salt until what you add remains free flowing (no clumping). After about 10-15 min remove the Na2SO4 solids by gravity filtration (no vacuum; use regular funnel with folded larger filter paper) into a new flask. Rinse the flask and any drying agent into the filter with a small amount of diethyl ether.
Add 15 mL of ligroin (66-77oC ligroin; ligroin is an organic solvent sometimes referred to as pertroleum ether, but it is not an ether chemically) and concentrate the diethyl ether-liqroin solution using a heating plate in the hood, to vent the ether. Because diethyl ether has a low boiling point (35oC), it will preferentially be removed compared to the ligroin. Continue to slowly evaporate the ethers until crystals of triphenylcarbinol (another name for triphenylmethanol) just begin to form. Remove the flask from the heating hot plate and let the flask cool slowly to room temperature. Crystals should be apparent. After reaching room temperature with the flask sitting on the bench top, you can then place the flask in an ice bath, but only after the flask has reached room temperature. Let the flask sit on ice until no more crystals form (10-15 min).
Perform a vacuum filtration and collect your crystals. You should wet the filter paper in the Büchner funnel with ligroin (do not use water!). After collecting most of your crystals, you can wash the remainder into the funnel using ligroin (not water). Concentration of the mother liquor (the filtrate) may yield a second crop of crystals, but this is not necessary. If you desire to increase your yield, simply take the filtrate and evaporate more of the ligroin until you start to see crystals again. Cool to room temperature, place on ice, and filter again. Combine crystals from the second crystallization (if you do it) with the first batch of crystals. Store the crystals in the drying oven until the next lab period.
STOP AND STORE THIS PART OF THE EXPERIMENT UNTIL THE NEXT LAB PERIOD!
To purify your collected benzoic acid from your first day, add about 10 mL of diethyl ether and after swirling to mix and dissolve the acid, simply transfer all of your material into a Separatory funnel. You can wash your container with a little diethyl ether which should dissolve any remaining benzoic acid crystals. Add about 10 mL of diethyl ether and about 10 mL of DI water to your Separatory funnel. Shake your funnel and let the two phases separate. Both liquid phases should be clear. Remove the lower aqueous phase, which will be discarded (as a precaution, keep all material until you are sure you have the correct phase with your benzoic acid in it). If two phases are not readily apparent, or if phases are not clear, you should add a little more diethyl ether and maybe a little HCl. After the aqueous phase has been removed, wash the top ether phase, which contains dissolved benzoic acid, with about 10 mL 1 M NaOH, which will convert the benzoic acid into the benzoate ion, which in turn will partition into the lower aqueous (basic) phase. Collect and save the lower layer (your chemical product is in this lower phase so do not discard). Repeat the 1 M NaOH extraction one more time. Decant the lower aqueous layer for a second time and combine with the previous extraction (this second wash removes any dissolved benzoate ion from the ether phase). (Use minimal amounts of wash solution. Each NaOH wash should contain less than 10 mL of NaOH solution. Keep your wash volumes small, to allow for optimal crystallization of the benzoic acid.)
Take the NaOH phase from the washes above and isolate benzoic acid from it. To facilitate benzoic acid formation, add enough 6 M HCl to make the solution acidic, checking that the pH is about 1 is achieved using pHydroin paper to verify. Cool your acidic mixture (to reduce the amount of dissolved benzoic acid) and collect the solid by vacuum filtration. Use ice cold water to wash the container of benzoic acid crystals during the filtration step.
Allow the crystals to dry thoroughly until the next lab period in the drying oven.
STOP AND STORE THIS PART OF THE EXPERIMENT UNTIL THE NEXT LAB PERIOD!
Weigh the dry triphenylmethanol from Day 2. The product should be colorless or whitish in appearance. Determine a melting point, which should be below 160oC. A typical yield should be close to 2.0 g. How well did you do?
Weigh the dry benzoic acid from Day 2. The product should be colorless or whitish in color. Determine a melting point, which should be close to 122oC. A typical yield should be close to 1.0 g. How well did you do?
You will need to determine percent yield, based on the appropriate starting material.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Bromobenzene | 157.02 | 4.5 mL (6.75 g) (use pipettor) | 43.0 | -31 | 130 | 1.491 | 1.5590 | msds |
| Diethyl ether, dry | 74.12 | ~80-100 mL total | 34.5 | 0.708 | 1.3530 | msds | ||
| Tetrahydrofuran (THF) | 72.11 | -108.3 | 65 | 0.8892 | msds | |||
| Phenylmagnesium bromide | 181.31 | 15.0 mL | 15.0 | msds | ||||
| Magnesium | 24.3 | 1.0 g | 41.0 | msds | ||||
| Benzophenone | 182.21 | 2.0 g | 11.0 | 48 | 305 | 1.1108 | msds | |
| Benzoic acid | 122.1232 | --- | --- | 122.4 | --- | 1.08 | msds | |
| Triphenylmethanol | 260.34 | 163 | 360 | msds | ||||
| Biphenyl | 154.21 | 72 | 255 | 0.992 | msds | |||
| (Iodine) | 253.81 | ~3-5 small crystals | 113 | 184 | 4.930 | msds | ||
| (Dibromoethane) | 187.87 | 20 drops | 10 | 133 | 2.180 | 1.5385 | msds | |
| Ligroin (petroleum ether) | --- | msds | ||||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
NOTES:
QUESTIONS
Go To Experiment: ChemDraw 1
2
3 4
5 6
7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright Donald L. Robertson (Date last modified: 11/14/2012)