 |
Performing an Aldol Condensation seeing how a molecule like acetone with two alpha-carbons can be used to bridge an aldehyde (benzaldehyde) in two reaction steps. A ketone is produced, but when the b-hydroxy carbonyl initial product of the Aldol Condensation undergoes dehydration, you produce a multipli-conjugated double-bond system.
An aldol condensation is a reaction that is named based on the type of product formed when two aldehydes (or ketones), in the presence of dilute base, yields a molecule having both aldehyde (ald-) and alcohol (-ol) functional groups. The aldol products are β-hydroxyaldehydea (or β-hydroxyketonea). This reaction is used extensively for the synthesis of new C-C bonds and to make larger organic molecules. In every case, the product results from the addition of one molecule of an aldehyde (or ketone) to a second aldehyde (or ketone) in such a way that the α-carbon (in the form of an enolate ion) of the first becomes attached to the carbonyl carbon of the second. This reaction is depicted below.
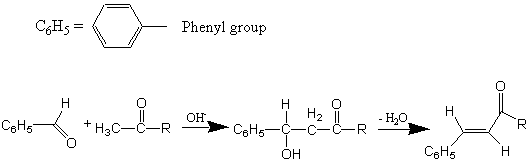 |
Although a β-hydroxyaldehyde (or a β-hydroxyketone) is produced in an aldol condensation, the ultimate product of these reactions (as shown above) is usually the α,β-unsaturated aldehyde (or ketone) and a separate molecule of water. Upon heating, the β-hydroxy aldehyde (or ketone) product of an aldol condensation easily undergoes dehydration to yield an α,β-unsaturated aldehyde (or ketone). Conjugation of the newly formed double bond with the carbonyl group (or of the benzene ring, as shown below) stabilizes the unsaturated product and provides the thermodynamic driving force for the dehydration process.
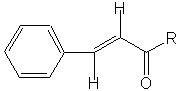
| Showing the a,b-unsaturated Aldol Condensations produce. |
In the present case, the reaction?a mixed, or crossed aldol condensation involving an aromatic aldehyde?is referred to as a Claisen-Schmidt condensation. The Claisen-Schmidt condensation always involves dehydration of the mixed aldol condensation product to yield a chemical in which the double bond (produced during dehydration) is conjugated to both the aromatic ring and the carbonyl group. Because this aromatic aldehyde lacks α-hydrogens, only one product is can be formed, rather than a mixture of four different compounds, as long as the concentration of the second aldehyde is carefully controlled. We will prepare the dibenzalacetone: 1,5-diphenyl-1,4-pentadien-3-one. The equilibrium is shifted toward the product because the compound precipitates from the reaction mixture as it is formed.
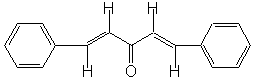 |
1,5-diphenyl-1,4-pentadien-3-one |
In a 50-mL round-bottom flask, containing a curved magnetic stirring bar (clam-shell-shaped stirring bar), attach a condenser column for reflux (just to keep volatile chemicals from evaporating). Combine the benzaldehyde (2.40 mL; use pipettor) with 0.90 mL of acetone (reagent grade; use pipettor) in the round-bottom flask. To this reaction mixture, you will then need to add 25 mL of aqueous ethanolic sodium hydroxide (formulation is given below; sodium ethoxide solution).
The reaction mixture is stirred at room temperature for 30 min. During this time a yellow solid precipitate should form in the flask. If a precipitate does not form, there would likely be a heavy yellow oil. This oil can be solidified by stirring it vigorously with a spatula or stirring rod. Be patient! Let it stir for an hour more, if necessary for solid to form. If no solid forms, then you will need to repeat the reaction from the beginning because you probably got something into the reaction mixture that simply prevent reaction from occurring. However, as a last resort, you could draw off the basic aqueous layer (is this the upper or lower layer?) and stir the remaining organic layer (the oil-like layer) vigorously. Cool the mixture on ice, but do not add this water to the reaction mixture. If solid does not from, which usually always occurs, just repeat the experiment (or take your neighbor's solid and pretend it is yours! Just kidding.
Collect the crude product using vacuum filtration in a Büchner funnel. Any solid remaining in the reaction container can be washed out using a small quantity of cold water (water does not dissolve your product). Wash the precipitate with a small amount of water to remove any chemicals soluble in water from the reaction mixture.
Transfer the solid product to a beaker. Add about 20 mL of 5% acetic acid in ethanol. Stir the solid suspension, which will not dissolve, and filter this washed mixture through a Büchner funnel. Wash the solid collected in the funnel with a small amount of cold ethanol.
STOP, label (with student names, name of chemical, experiment, and other required items) and store your solid material in the drying oven until the next lab period. The dried material will be used for additional purification and recrystallization.
The dry product from the previous lab period will be recrystallized by using about 20 mL of hot, boiling 95% ethanol. After dissolving, crystals should reform as soon as the solution cools to room temperature (without the aid of ice). You should be careful to dissolve your solid dibenzalacetone in a minimum volume of the hot (boiling) 95% ethanol. Many students use too much solvent, thinking that the product must be dissolved in warm (not hot) 95% ethanol. If too much ethanol is used, and no crystals form, it will be necessary to reduce the volume to about 15 mL by evaporation. (Alternatively, ethyl acetate can be use as the recrystallization solvent if you choose.)
Collect the crystallized solid using filtration using a Büchner funnel as before. Since you are usually a volatile organic solvent for crystallization, it is not necessary to dry your sample overnight. Simply allow your solid material to dry in the Büchner funnel for 10-15 min, with vacuum on. This will allow air to continue to be drawn across your solid and evaporate your solvent.
Determine the mass of your dry product and determine percent yield. What reactant is your percent yield based on? Determine the melting point and compare to the literature value. If requested by your instructor, obtain an IR using the Nujol mull technique and identify pertinent peaks.
| Compound | MW | Amount | mmol | mp | bp | Density | ηD | msds |
|---|---|---|---|---|---|---|---|---|
| Benzaldehyde1 | 106.13 | 2.40 mL (use pipettor) | 23.5 | -56oC | 178oC | 1.04 | 1.5463 | msds |
| Acetone (reagent) | 58.08 | 0.90 mL (use pipettor) | 12.2 | 56oC | 0.79 | 1.3590 | msds | |
| NaOH in H2O/EtOH2 | 25.0 mL | msds | ||||||
| 5% acetic acid/EtOH3 | 20.0 mL | msds | ||||||
| 95% Ethanol | 20.0 mL | msds | ||||||
| Dibenzalacetone | 234.30 | 107oC | ||||||
| Compound | g/mol | grams or mL | 10-3 mol | oC | oC | g/mL | ηD | msds |
1Toxic/irritant - suspected carcinogen
2Corrosive; This basic solution is the catalyst. Each group will need 25 mL of this solution. The stock reagent for the entire lab is made by dissolving 24.0 g NaOH pellets in 240 mL of water and, after the pellets are dissolved, adding 180 mL of 95% ethanol. Make immediately before use. If a lab stock solution is not prepared, or it has been used, each student group can prepare his own solution by dissolving 1.8 g NaOH in 18.0 mL water and then adding 13.5 mL 95% ethanol. (Excess solution should be disposed of properly after the lab.)
3For 15 groups, use 15 mL glacial acetic and 285 mL 95% ethanol (300 mL total).
Go To Experiment: ChemDraw 1
2
3 4
5 6
7 8
9 10
Return to Chem211 Experiment Protocols Index
Copyright Donald L. Robertson (Date last modified: 11/14/2012)